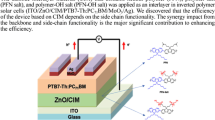
Abstract
Three alcohol/water-soluble porphyrins (Zn-TPyPMeI:zinc(II) meso-tetra(N-methyl-4-pyridyl) porphyrin tetra-iodide, Zn-TPyPAdBr:zinc(II) meso-tetra[1-(1-adamantylmethyl ketone)-4-pyridyl] porphyrin tetra-bromide and MnCl-TPyPAdBr:man-ganese(III) meso-tetra[1-(1-adamantylmethyl ketone)-4-pyridyl] porphyrin tetra-bromide were employed as cathode interlayers to fabricate polymer solar cells (PSCs). The PC71BM ([6,6]-phenyl C71 butyric acid methyl ester) and PCDTBT (poly[N-9″-hepta-decanyl-2,7-carbazole-alt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)])-blend films were used as active layers in polymer solar cells (PSCs). The PSCs with alcohol/water-soluble porphyrins interlayer showed obviously higher power conversion efficiency (PCE) than those without interlayers. The highest PCE, 6.86%, was achieved for the device with MnCl-TPyPAdBr as an interlayer. Ultraviolet photoemission spectroscopic (UPS), carrier mobility, atomic force microscopy (AFM) and contact angle (θ) characterizations demonstrated that the porphyrin molecules can result in the formation of interfacial dipole layer between active layer and cathode. The interfacial dipole layer can obviously improve the open-circuit voltage (V oc) and charge extraction, and sequentially lead to the increase of PCE.
Similar content being viewed by others
References
Chen HY, Hou JH, Zhang SQ, Liang YY, Yang GW, Yang Y, Yu LP, Wu Y, Li G. Polymer solar cells with enhanced open circuit voltage and efficient. Nat Photonic, 2009, 3: 649–653
Ye L, Zhang SQ, Zhao WC, Yao HF, Hou JH. Highly efficient 2D-conjugated benzodithiophene-based photovoltaic polymer with linear alkylthio side chain. Chem Mater, 2014, 26: 3603–3605
He ZC, Zhong CM, Su SJ, Xu M, Wu HB, Cao Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat Photonic, 2012, 6: 591–595
He ZC, Zhong C, Huang X, Wong WY, Wu H, Chen L, Su S, Cao Y. Simultaneous enhancement of open-circuit voltage, short-circuit current density, and fill factor in polymer solar cells. Adv Mater, 2011, 23: 4636–4643
Liang Y, Xu Z, Xia J, Tsai ST, Wu Y, Li G, Ray C, Yu L. For the bright future—bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv Mater, 2010, 22: E135–E138
Huo L, Zhang S, Guo X, Xu F, Li Y, Hou J. Replacing alkoxy groups with alkylthienyl groups: a feasible approach to improve the properties of photovoltaic polymers. Angew Chem Int Ed, 2011, 50: 9697–9702
Deng Y, Liu J, Wang J, Liu L, Li W, Tian H, Zhang X, Xie Z, Geng Y, Wang F. Dithienocarbazole and isoindigo based amorphous low bandgap conjugated polymers for efficient polymer solar cells. Adv Mater, 2013, 3: 471–476
Gao L, Zhang J, He C, Zhang Y, Sun QJ, Li YF. Effect of additives on the photovoltaic properties of organic solar cells based on triphen-ylamine-containing amorphous molecules. Sci China Chem, 2014, 57: 966–972
Liu X, Cai P, Chen DC, Chen JW, Su SJ, Cao Y. Small molecular non-fullerene electron acceptors for P3HT-based bulk-heterojunction solar cells. Sci China Chem, 2014, 57: 973–981
Liu J, Shao S, Fang G, Meng B, Xie Z, Wang L. High-efficiency inverted polymer solar cells with transparent and work-function tunable MoO3-Al composite film as cathode buffer layer. Adv Mater, 2012, 24: 2774–2779
Yang TB, Qin DH, Lan LF, Huang WB, Gong X, Peng JB, Cao Y. Inverted polymer solar cells with a solution-processed zinc oxide thin film as an electron collection layer. Sci China Chem, 2012, 55: 755–759
Jo J, Na SI, Kim SS, Lee TW, Chung Y, Kang SJ, Vak D, Kim DY. Three-dimensional bulk heterojunction morphology for achieving high internal quantum efficiency in polymer solar cells. Adv Funct Mater, 2009, 19: 2398–2406
Tang Z, Andersson LM, George Z, Vandewal K, Tvingstedt K, Heriksson P, Kroon R, Andersson MR, Inganäs O. Interlayer for modified cathode in highly efficient inverted ITO-free organic solar cells. Adv Mater, 2012, 24: 554–558
Oh SH, Na SI, Jo J, Lim B, Vak D, Kim DY. Water-soluble polyfluorenes as an interfacial layer leading to cathode-independent high performance of organic solar cells. Adv Funct Mater, 2010, 20: 1977–1983
Zhao Y, Xie Z, Qin C, Qu Y, Geng Y, Wang L. Enhanced charge collection in polymer photovoltaic cells by using an enthanol-soluble conjugated polyfluorene as cathode buffer layer. Sol Energ Mat Sol C, 2009, 93: 604–608
Seo JH, Gutacker A, Sun Y, Wu H, Huang F, Cao Y, Scherf U, Heeger AJ, Bazan GC. Improved high-efficiency organic solar cells via incorporation of a conjugated polyelectrolyte interlayer. J Am Chem Soc, 2011, 133: 8416–8419
Chang YM, Zhu R, Richard E, Chen CC, Li G, Yang Y. Electrostatic self-assembly conjugated polyelectrolyte-surfactant complex as an interlayer for high performance polymer solar cells. Adv Funct Mater, 2012, 22: 3284–3289
Li SS, Lei M, Lv ML, Watkins SE, Tan ZA, Zhu J, Hou JH, Chen XW, Li YF. [6,6]-Phenyl-C61-butyric acid dimethylamino ester as a cathode buffer layer for high-performance polymer solar cells. Adv Energy Mater, 2013, 3: 1569–1574
Ye H, Hu X, Jiang Z, Chen D, Liu X, Nie H, Su SJ, Gong X, Cao Y. Pyridinium salt-based molecules as cathode interlayers for enhanced performance in polymer solar cells. J Mater Chem A, 2013, 1: 3387–3394
Vasilopoulou M, Georgiadou DG, Douvas AM, Soultati A, Constantoudis V, Davazoglou D, Gardelis S, Palilis LC, Fakis M, Kennou S, Lazarides T, Coutsolelos AG, Argitis P. Porphyin oriented self-assembled nanostructures for efficient exciton dissociation in high-performing organic photovoltaics. J Mater Chem A, 2014, 2: 182–192
Zhou J, Wan X, Liu Y, Zuo Y, Li Z, He G, Long G, Ni W, Li C, Su X, Chen Y. Small molecules based on benzo[1,2-b:4,5-b′]dithiophene unit for high-performance solution-processed organic solar cells. J Am Chem Soc, 2012, 134: 16345–16351
Tsuda A, Osuka A. Fully conjugated porphyrin tapes with electronic absorption bands that reach into infrared. Science, 2001, 293: 79–82
Zhang H, Zhang B, Zhu M, Grayson SM, Schmehl R, Jayawickramarajah J. Water-soluble porphyrin nanospheres: enhancedphoto-physical properties achieved viacyclodextrin driven double self-inclusion. Chem Commun, 2014, 50: 4853–4855
Dong RJ, Bo Y, Tong G, Zhou Y, Zhu X, Lu Y. Self-assembly and optical properties of a porphyrin-based amphiphile. Nanoscale, 2014, 6: 4544–4550
Huo C, Zhang HD, Zhang HY, Zhang HY, Yang B, Zhang P, Wang Y. Synthesis and assembly with mesoprous silica MCM-48 of platinum prophyrin complexes bearing carbazeyl groups: spectroscopic and oxygen sensing properties. Inorg Chem, 2006, 45: 4735–4742
Bhyrappa P, Young JK, Moore JS, Suslick KS. Dendrimer-metallo-porphyrins: synthesis and catalysis. J Am Chem Soc, 1996, 118: 5708–5711
Fateeva A, Chater PA, Ireland CP, Tahir AA, Khimyak YZ, Wiper PV, Darwent JR, Rosseinsky MJ. A water-stable porphyrin-based metal-organic framework active for visible-light photocatalysis. Angew Chem Int Ed, 2012, 124: 7558–7562
Janghouri M, Mohajerani E, Amini MM, Safari N. Porphyrin doping of dichloride-bis(5,7-dichloroquinolin-8-olato)tin (IV) complex for electroluminescence. J Porphyr Phthalocya, 2013, 17: 351–358
Graham KR, Yang Y, Sommer JR, Shelton AH, Schanze KS, Xue J, Reynolds JR. Extended conjugation platinum prophyrins for use in near-infrared emitting organic light emitting diodes. Chem Mater, 2011, 23: 5305–5312
Mathew S, Yella A, Gao P, Humphry-Baker R, Curchod BE, Ashari-Astani N, Tavernelli I, Rothlisberger U, Nazeeruddin MK, Grtäzel M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat Chem, 2014, 6: 242–247
Luechai A, Gasiorowski J, Petsom A, Neugebauer H, Sariciftci NS, Thamyongkit P. Photosensitizing porphyrin-triazine compound for bulk heterojunction solar cells. J Mater Chem, 2012, 22: 23030–23037
Singh VK, Kanaparthi RK, Giribabu L. Emerging molecular design strategies of unsymmetrical phthalocyanines for dye-senstitized solar cell applications. RSC Adv, 2014, 4: 6970–6984
Wu C, Chen M, Su P, Kuo H, Wang C, Lu C, Tsai C, Wu C, Lin C. Porphyrins for efficient dye-sensitized solar cells covering the near-IR region. J Mater Chem A, 2014, 2: 991–999
Zervaki GE, Papastamatakis E, Angaridis PA, Nikolaou V, Singh M, Kurchania R, Kitsopoulos TN, Sharma GD, Coutsolelos AG. A propeller-shaped, triazine-linked porphyrin triad as efficient sensitizer for dye-sensitized solar cells. Eur J Inorg Chem, 2014, 6: 1020–1033
Vasilopoulou M, Georgiadou DG, Douvas AM, Soultati A, Constantoudis V, Davazoglou D, Gardelis S, Palilis LC, Fakis M, Kennou S, Lazarides T, Coutsolelosd AG, Argitis P. Porphyrin oriented self-assembled nanostructures for efficient exciton dissociation in high-performing organic photovoltaics. J Mater Chem A, 2014, 2: 182–192
Zervaki GE, Roy MS, Panda MK, Angaridis PA, Chrissos E, Sharma GD, Coutsolelos AG. Efficient sensitization of dye-sensitized solar cells by novel triazine-bridged porphyrin-porphyrin dyads. Inorg Chem, 2013, 52: 9813–9825
Sharmaa GD, Daphnomili D, Biswas S, Coutsolelos AG. New soluble porphyrin bearing a pyridinylethynyl group as donor for bulk heterojunction solar cells. Org Electronics, 2013, 14: 1811–1819
Choi S, Chae SH, Hoang MH, Kim KH, Huh JA, Kim Y, Kim SJ, Choi DH, Lee SJ. An unsymmetrically π-extended porphyrin-based single-crystal field-effect transistor and its anisotropic carrier-transport behavior. Chem Eur J, 2013, 19: 2247–2251
Zhang ZG, Li H, Qi BY, Chi D, Jin ZW, Qi Z, Hou JH, Li YF, Wang JZ. Amine group functionalized fullerene derivatives as cathode buffer layers for high performance polymer solar cells. J Mater Chem A, 2013, 1: 9624–9629
Zhang ZG, Hui H, Qi Z, Jin ZW, Liu G, Hou JH, Li YF, Wang JZ. Poly(ethylene glycol) modified [60]fullerene as electron buffer layer for high-performance polymer solar cells. Appl Phys Lett, 2013, 102: 143902
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, T., Zhou, W., Li, F. et al. Alcohol/water-soluble porphyrins as cathode interlayers in high-performance polymer solar cells. Sci. China Chem. 58, 323–330 (2015). https://doi.org/10.1007/s11426-014-5218-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-014-5218-4