Abstract
Purpose
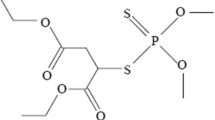
There is very little information about the toxicological and pathological effects of synthetic cannabinoids, which have cannabis-like properties. This study was carried out to histopathologically, hematologically, and biochemically determine the toxic effects of acute and subacute exposure to a novel synthetic cannabinoid 1-(4-cyanobutyl)-N-(2-phenylpropan-2-yl)indazole-3-carboxamide in internal organs of adult male rats.
Methods
The cannabinoid was injected intraperitoneally at three doses (0.5, 1.0, and 2.0 mg/kg, body weight). The cannabinoid was administered to acute groups for 2 days and to subacute groups for 14 days. Observations were made for 14 days and various changes such as mortality, injury, and illness were recorded daily. Hematological and biochemical changes were evaluated and histopathological analyses in lung, liver, and kidney tissues were also performed.
Results
No mortality was observed. It was observed that there were fluctuations in hematological and serum biochemical parameters. Among the oxidative stress parameters, significant decreases in superoxide dismutase, catalase levels and significant increases in lipid peroxidation levels were determined. Serious pathological changes such as necrosis, vacuolation, congestion, and fibrosis were observed in the internal organs in a dose-dependent and time-dependent manner. It was also found that the synthetic cannabinoid triggered apoptosis in the organs. The results demonstrated that the most affected organ by the cannabinoid was the kidney.
Conclusion
This study showed for the first time that CUMYL-4CN-BINACA adversely affects healthy male albino rats. It can be estimated that the abuse of the cannabinoid may harm human health in the same way.






Similar content being viewed by others
References
Dignam G, Bigham C (2017) Novel psychoactive substances: a practical approach to dealing with toxicity from legal highs. BJA Education 17(5):172–177. https://doi.org/10.1093/bjaed/mkw068
The European Monitoring Centre for Drugs and Drug Addiction (2022) Trends and Developments. Available online: https://www.emcdda.europa.eu/publications/edr/trends-developments/2022_en (Accessed on 12 May 2023).
Cooper ZD (2016) Adverse effects of synthetic cannabinoids: management of acute toxicity and withdrawal. Curr Psychiatry Rep 18:52. https://doi.org/10.1007/s11920-016-0694-1
Hermanns-Clausen M, Müller D, Kithinji J, Angerer V, Franz F, Eyer F, Neurath H, Liebetrau G, Auwärter V (2018) Acute side effects after consumption of the new synthetic cannabinoids AB-CHMINACA and MDMB-CHMICA. Clinical Toxicol 56(6):404–411. https://doi.org/10.1080/15563650.2017.1393082
Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V (2013) Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction (Abingdon, England) 108(3):534–544. https://doi.org/10.1111/j.1360-0443.2012.04078.x
Schaefer N, Peters B, Bregel D, Kneisel S, Auwärter V, Schmidt PH, Ewald AH (2013) A fatal case involving several synthetic cannabinoids. Toxichem Krimtech 80:248–251
Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S (2016) A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol 54:1–13. https://doi.org/10.3109/15563650.2015.1110590
Madras B, Kuhar M (2013) The effects of drug abuse on the human nervous system. Elsevier, Oxford, UK
Alzub’i A, Zoubi MSA, Al-Trad B, AbuAlArjah MI, Shehab M, Alzoubi H, Albals D, Abdelhady GT, El-Huneidi W (2022) Acute hepatic injury associated with acute administration of synthetic cannabinoid XLR-11 in mouse animal model. Toxics 10(11):668. https://doi.org/10.3390/toxics10110668
Koller VJ, Zlabinger GJ, Auwarter V, Fuchs S, Knasmueller S (2013) Toxicological profiles of selected synthetic cannabinoids showing high binding affinities to the cannabinoid receptor subtype CB(1). Arch Toxicol 87(7):1287–1297. https://doi.org/10.1007/s00204-013-1029-1
Roque-Bravo R, Silva RS, Malheiro RF, Carmo H, Carvalho F, da Silva DD, Silva JP (2023) Synthetic cannabinoids: a pharmacological and toxicological overview. Annu Rev Pharmacol Toxicol 63:187–209. https://doi.org/10.1146/annurev-pharmtox-031122-113758
Drug Enforcement Administration, Department of Justice (2018) Schedules of controlled substances: temporary placement of NM2201, 5F-AB-PINACA, 4-CN-CUMYL-BUTINACA, MMB-CHMICA and 5F-CUMYL-P7AICA into schedule I. Fed Regist 83:24696–24701
The European Monitoring Centre for Drugs and Drug Addiction (2018) Report on the risk assessment of 1-(4-cyanobutyl)-N-(2-phenylpropan-2-yl)- 1H-indazole-3-carboxamide in the framework of the council decision on new psychoactive substances. Available online: https://www.emcdda.europa.eu/publications/risk-assessments/cumyl-4cn-binaca_en (Accessed on 12 May 2023).
Järbe TUC, Raghav JG (2017) Tripping with synthetic cannabinoids (“Spice”): anecdotal and experimental observations in animals and man. Curr Top Behav Neurosci 32:263–281. https://doi.org/10.1007/7854201616
World Health Organization (2018) Critical review report: CUMYL-4CN-BINACA. Expert committee on drug dependence forty-first meeting Geneva, 12–16 November 2018. https://ecddrepository.org/sites/default/files/2023-04/cumyl_4cn_binaca.pdf (Accessed on 12 May 2023).
Diao X, Huestis MA (2019) New synthetic cannabinoids metabolism and strategies to best identify optimal marker metabolites. Front Chem 7:109–109. https://doi.org/10.3389/fchem.2019.00109
Gatch MB, Forster MJ (2018) Δ 9-Tetrahydrocannabinol-like discriminative stimulus effects of five novel synthetic cannabinoids in rats. Psychopharmacology 235(3):673–680. https://doi.org/10.1007/s00213-017-4783-6
Kevin RC, Anderson L, Mcgregor IS, Boyd R, Manning JJ, Glass M, Connor M, Banister SD (2019) CUMYL-4CN-BINACA is an efficacious and potent pro-convulsant synthetic cannabinoid receptor agonist. Front Pharmacol 10:595. https://doi.org/10.3389/fphar.2019.00595
Organization for economic cooperation and development (2002). OECD 423: TG for acute oral toxicity ‒ acute toxic class method; adopted 22 March 1996, last updated 8 Feb 2002.
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 1(2):87–93. https://doi.org/10.4103/0976-500X.72350
Gu X, Herrera GA (2010) Expression of eNOS in kidneys from hypertensive patients. Int J Nephrol Renovasc Dis 3:11–19. https://doi.org/10.2147/ijnrd.s6572
Huang JS, Yang CM, Wang JS, Liou HH, Hsieh IC, Li GC, Huang SJ, Shu CW, Fu TY, Lin YC, Ger LP, Liu PF (2017) Caspase-3 expression in tumorigenesis and prognosis of buccal mucosa squamous cell carcinoma. Oncotarget 8(48):84237–84247. https://doi.org/10.18632/oncotarget.20494
Noor KK, Ijaz MU, Ehsan N, Tahir A, Yeni DK, Neamul Kabir Zihad SM, Uddin SJ, Ashraf A, Simal-Gandara J (2022) Hepatoprotective role of vitexin against cadmium-induced liver damage in male rats: a biochemical, inflammatory, apoptotic and histopathological investigation. Biomed Pharmacother 150:112934. https://doi.org/10.1016/j.biopha.2022.112934
Seely KA, Lapoint J, Moran JH, Fattore L (2012) Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry 392:234–243. https://doi.org/10.1016/j.pnpbp.2012.04.017
Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K (2012) Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med 30(7):1320 e5-1327. https://doi.org/10.1016/j.ajem.2011.05.013
Forrester MB, Kleinschmidt K, Schwarz E, Young A (2012) Synthetic cannabinoid and marijuana exposures reported to poison centers. Hum Exp Toxicol 31(10):1006–1011. https://doi.org/10.1177/0960327111421945
World Health Organization (2020) WHO Expert Committee on Drug Dependence: forty-first report. World Health Organization. Available at: https://www.who.int/publications/i/item/ 9789241210270
Wei X, Zhang J, Niu J, Zhou X, Li J, Li B (2015) Evaluation of arecoline hydrobromide toxicity after a 14-day repeated oral administration in Wistar rats. PLoS ONE 10(4):e0120165
Brown G (1992) Haematology tests in toxicology: time for a re-think? Comp Haematol Intern 2:231–235
Evans GO (2008) Animal Hematotoxicology: a practical guide for toxicologists and biomedical researchers. CRC Press, Boca Raton, FL
DeBonis K, Pierre JM (2011) Psychosis, ivermectin toxicity, and" Morgellons disease". Psychosomatics 52(3):295–296
Adeoye GO, Alimba CG, Oyeleke OB (2015) The genotoxicity and systemic toxicity of a pharmaceutical effluent in Wistar rats may involve oxidative stress induction. Toxicol Reports 2:1265–1272
Sharma S, Sharma A, Singh PK, Soni P, Sharma S, Sharma P, Sharma KP (2007) Impact of distillery soil leachate on haematology of Swiss albino mice (Mus musculus). Bull Environ Contam Toxicol 79:273–277
Obonga WO, Osadebe PO, Esimone CO, Ihedioha J (2005) Haematotoxic evaluation of crude cannabis resin in rats. J Pharma Allied Sci 3:278–282
Obembe AO, Omini GC, Okon UA, Okpo-ene AI, Ikpi DE (2015) Hematological and immunological effect of Cannabis sativa on albino wistar rats. Br J Med Med Res 7(1):52
Bayramer H (2017) Determination of the effects of synthetic cannabinoids (JWH-200) on hematological parameters of rats. Mehmet Akif Ersoy University, Health Sciences Institute. Master Thesis. Burdur, Turkey (in Turkish with English anstract).
Dziwenka M, Coppock R, Alexander M, Palumbo E, Ramirez C, Lermer S (2020) Safety assessment of a hemp extract using genotoxicity and oral repeat-dose toxicity studies in Sprague-Dawley rats. Toxicol Reports 7:376–385. https://doi.org/10.1016/j.toxrep.2020.02.014
Ewing LE, Skinner CM, Quick CM, Kennon-McGill S, McGill MR, Walker LA, ElSohly MA, Gurley BJ, Koturbash I (2019) Hepatotoxicity of a Cannabidiol-rich Cannabis extract in the mouse model. Molecules (Basel, Switzerland) 24(9):1694. https://doi.org/10.3390/molecules24091694
López-Malo D, Sanchez-Martinez JJ, Romero FJ, Barcia JM, Villar VM (2016) Oxidative stress and the combined use of tetrahydrocannabinol and alcohol: is there a need for further research? React Oxyg Species 2(6):388–395
Nafea OE, ElKhishin IA, Awad OA, Mohamed DA (2016) A study of the neurotoxic effects of tramadol and Cannabis in adolescent male albino rats. Int J Sci Rep 2(7):143–154
Sezer Y, Jannuzzi AT, Huestis MA, Alpertunga B (2020) In vitro assessment of the cytotoxic, genotoxic and oxidative stress effects of the synthetic cannabinoid JWH-018 in human SH-SY5Y neuronal cells. Toxicol Res 9(6):734–740. https://doi.org/10.1093/toxres/tfaa078
Abdelmoneim WM, Bakr MH, Ghandour NM, Mohammed MK, Fawzy M, Ramadan AG, Abdellah NZ (2023) Cytotoxicity associated with acute and chronic administration of synthetic cannabinoids “Strox” in the brain, liver, heart, and testes of male albino rats: histological and immunohistochemical study. Egypt J Forensic Sci 13:1–21. https://doi.org/10.1186/s41935-023-00331-8
Abass M, Hassan M, Abd Elhaleem M, Abd Elaziz H, Abd-Allah R (2017) Acute toxicity of a novel class of hallucinogen. Ain Shams J Forensic Med Clin Toxicol 28(1):62–73. https://doi.org/10.21608/ajfm.2017.18280
Mousa R, Gebri S, Masoud K, Radwan R, Mohamad S (2021) Acute toxic effects of AB-CHMINACA on lung, heart and liver: an experimental pilot study. Ain Shams J Forensic Med Clin Toxicol 37(2):128–135. https://doi.org/10.21608/AJFM.2021.182715
Mohammad SA, Mousa REA, Gebril SM, Masoud KMM, Radwan RA (2023) Toxic effects of AB-CHMINACA on liver and kidney and detection of its blood level in adult male mice. Forensic Toxicol. https://doi.org/10.1007/s11419-023-00670-0. (Advanceonlinepublication.10.1007/s11419-023-00670-0)
ElAmin EHA, Albloi RH, BakdashA KS (2022) Single-dose acute toxicity of 4-F-MDMB-BUTINACA designer cannabis drug: LD50 and histological changes in mice. Indian J Forensic Med Toxicol 16(3):145–152
Zawatsky CN, Abdalla J, Cinar R (2020) Synthetic cannabinoids induce acute lung inflammation via cannabinoid receptor 1 activation. ERJ Open Res 6(3):00121–02020. https://doi.org/10.1183/23120541.00121-2020
Bailey ME, Fraire AE, Greenberg SD, Barnard J, Cagle PT (1994) Pulmonary histopathology in cocaine abusers. Hum Pathol 25:203–207
Alon MH, Saint-Fleur MO (2017) Synthetic cannabinoid induced acute respiratory depression: case series and literatüre review. Respir Med Case Rep 22:137–141. https://doi.org/10.1016/j.rmcr.2017.07.011
Alhadi S, Tiwari A, Vohra R, Gerona R, Acharya J, Bilello K (2013) High times, low sats: diffuse pulmonary infiltrates associated with chronic synthetic cannabinoid use. J Med Toxicol 9:199–206. https://doi.org/10.1007/s13181-013-0288-9
Omran GA, Abd Allah ES, Mohammed SA, El Shehaby DM (2023) Behavioral, biochemical and histopathological toxic profiles induced by sub-chronic cannabimimetic WIN55, administration in mice. BMC Pharmacol Toxicol 24(1):1–13. https://doi.org/10.1186/s40360-023-00644-3
Al-Alem S (2013) Nephrotoxicity effect of Cannabis sativa (Marijuana) plant on the male albino rats. Tikrit J Pure Sci 18(3):16–19
Osheke O, Moses A, Florence T, Eze D (2017) Biochemical and histological changes in kidney and heart of male rats following oral administration of aqueous and methanolic extract of Cannabis sativa leaves. Eur J Biomed Pharm Sci 4:59–67
Proskuryakov SY, Konoplyannikov AG, Gabai VL (2003) Necrosis: a specific form of programmed cell death? Exp Cell Res 283(1):1–16
Festjens N, Vandenerghe T, Vandenabeele P (2006) Necrosis, a well-orchestrated form of cell demise: signalling cascades important mediators and concomitant immune response. Biochim Biophys Acta (BBA)-Bioenergetics. 1757(9–10):1371–1387. https://doi.org/10.1016/j.bbabio.2006.06.014
Goetz ME, Kunig G, Riederer P, Youdim MB (1994) Oxidative stress: free radical production in neural degeneration. Pharmacol Ther 63:37–122
Munoz-Luque J, Ros J, Fernandez-Varo G, Tugues S, Morales-Ruiz M, Alvarez CE, Friedman SL, Arroyo V, Jiménez W (2008) Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther 324(2):475–483. https://doi.org/10.1124/jpet.107.131896
Ali SM, Kolieb E, Imbaby S, Hagras AM, Korayem Arafat HE, Kamel EM, Abdelshakour MA, Mohammed Ali MI (2023) Acute toxic effects of new synthetic cannabinoid on brain: neurobehavioral and histological: preclinical studies. Chem Biol Interact 370:110306. https://doi.org/10.1016/j.cbi.2022.110306
Tomiyama KI, Funada M (2014) Cytotoxicity of synthetic cannabinoids on primary neuronal cells of the forebrain: the involvement of cannabinoid CB1 receptors and apoptotic cell death. Toxicol Appl Pharmacol 274:17–23. https://doi.org/10.1016/j.taap.2013.10.028
Pozzoli G, Tringali G, Vairano M, D’Amico M, Navarra P, Martire M (2006) Cannabinoid agonist WIN55,212–2 induces apoptosis in cerebellar granule cells via activation of the CB1 receptor and downregulation of bcl-xL gene expression. J Neurosci Res 83:1058–1065
Tomiyama KI, Funada M (2021) Synthetic cannabinoid CP-55,940 induces apoptosis in a human skeletal muscle model via regulation of CB 1 receptors and l-type Ca2+ channels. Arch Toxicol 95:617–630. https://doi.org/10.1007/s00204-020-02944-7
Silva JP, Araújo AM, de Pinho PG, Carmo H, Carvalho F (2019) Synthetic cannabinoids JWH-122 and THJ-2201 disrupt endocannabinoid-regulated mitochondrial function and activate apoptotic pathways as a primary mechanism of in vitro nephrotoxicity at in vivo relevant concentrations. Toxicol Sci 169(2):422–435. https://doi.org/10.1093/toxsci/kfz050
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Kamada S, Kikkawa U, Tsujimoto Y, Hunter T (2005) Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J Biol Chem 280:857–860
Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, WangChoi XAM (2007) Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9:49–89. https://doi.org/10.1089/ars.2007.9.49
Acknowledgements
This study was prepared from Ayşe Lafzi’s PhD thesis. The authors thank Dr. Cemil Bayram for his valuable supports.
Funding
This study was supported by the Atatürk University Research Fund (BAP, FCD-2021-9416).
Author information
Authors and Affiliations
Contributions
AL, FY, and TD performed the experiments. AH provided technical support. TŞ designed the study, analyzed data, and wrote the article. The published version of the manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript. We certify that the submission is original work and is not under review at any other publication.
Ethical approval
This study was approved by the Local Animal Ethics Committee of Atatürk University, on March 9, 2021 (no. 2021/40). All animal care procedures were performed according to the National Institutes of Health (NIH). Every effort was made to minimize the suffering of the animals during the experiment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lafzi, A., Yeşilyurt, F., Demirci, T. et al. Acute and subacute toxic effects of CUMYL-4CN-BINACA on male albino rats. Forensic Toxicol (2023). https://doi.org/10.1007/s11419-023-00676-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11419-023-00676-8