Abstract
Purpose
The presented study aimed to elucidate the toxicokinetics of the four synthetic cathinones 4-chloroethcathinone (4-CEC), N-ethylnorpentylone (N-ethylpentylone, ephylone), N-ethylhexedrone (NEH), and 4-fluoro-alpha-pyrrolidinohexiophenone (4-fluoro-alpha-pyrrolidinohexanophenone, 4-F-α-PHP, 4F-alpha-PHP, 4F-PHP).
Methods
First, their metabolism was studied using human urine and blood samples. Analysis of specimens was performed by liquid chromatography-high resolution tandem mass spectrometry (LC-HRMS/MS) and gas chromatography–mass spectrometry (GC–MS). LC-HRMS/MS was also used to analyze in vitro incubations of the new psychoactive substances using pooled human liver S9 fraction (pS9), to identify the monooxygenases involved in the initial metabolic steps, and determination of plasma concentrations after a standard addition method. Metabolic stability was tested in pooled human liver microsomes incubations analyzed by LC-ion trap MS.
Results
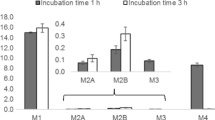
Using LC-HRMS/MS, 47 metabolites in total were found in patient samples and pS9 incubations. Using GC–MS, 4-CEC, ephylone, NEH, and five of their metabolites were detectable in urine. The following main phase I reactions were observed: carbonyl group reduction, N-deethylation, hydroxylation, lactam formation (4F-PHP), and demethylenation (ephylone). Mainly glucuronidations were observed as phase II reactions besides conjugates with the dicarboxylic acids malonic, succinic, and glutaric acid (4-CEC), sulfation, methylation (both ephylone), and N-acetylation (NEH). A broad range of monooxygenases was involved in the initial steps with exception of NEH (only CYP1A2 and CYP2C19). 4F-PHP had the shortest in vitro half-life (38 min) and highest intrinsic clearance (15.7 mL/min/kg). Plasma concentrations ranged from 0.8 to 8.5 ng/mL.
Conclusions
Our results are expected to help toxicologists to reliably identify these substances in case of suspected abuse and allow them a thorough risk assessment.




Similar content being viewed by others
References
UNODC. World Drug Report 2018. Booklet 3. Analysis of drug markets—opioids, cocaine, cannabis, synthetic drugs. In: UNODC, editor.: United Nations publication 2018
EMCDDA. European drug report 2018. Publications of of the European Union 2018
Tyrkko E, Andersson M, Kronstrand R (2016) The toxicology of new psychoactive substances: synthetic cathinones and phenylethylamines. Ther Drug Monit 38:190–216
Ellefsen KN, Concheiro M, Huestis MA (2016) Synthetic cathinone pharmacokinetics, analytical methods, and toxicological findings from human performance and postmortem cases. Drug Metab Rev 48:237–265
Krotulski AJ, Papsun DM, De Martinis BS, Mohr ALA, Logan BK (2018) N-ethyl pentylone (ephylone) intoxications: quantitative confirmation and metabolite identification in authentic human biological specimens. J Anal Toxicol 42:467–475
Helfer AG, Michely JA, Weber AA, Meyer MR, Maurer HH (2017) Liquid chromatography-high resolution-tandem mass spectrometry using Orbitrap technology for comprehensive screening to detect drugs and their metabolites in blood plasma. Anal Chim Acta 965:83–95
Wissenbach DK, Meyer MR, Remane D, Philipp AA, Weber AA, Maurer HH (2011) Drugs of abuse screening in urine as part of a metabolite-based LC-MSn screening concept. Anal Bioanal Chem 400:3481–3489
Maurer HH, Pfleger K, Weber AA (2016) Mass spectral data of drugs, poisons, pesticides, pollutants and their metabolites. Wiley-VCH, Weinheim
Richter LHJ, Maurer HH, Meyer MR (2017) New psychoactive substances: studies on the metabolism of XLR-11, AB-PINACA, FUB-PB-22, 4-methoxy-alpha-PVP, 25-I-NBOMe, and meclonazepam using human liver preparations in comparison to primary human hepatocytes, and human urine. Toxicol Lett 280:142–150
Chauret N, Gauthier A, Nicoll-Griffith DA (1998) Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug Metab Dispos 26:1–4
Wagmann L, Meyer MR, Maurer HH (2016) What is the contribution of human FMO3 in the N-oxygenation of selected therapeutic drugs and drugs of abuse? Toxicol Lett 258:55–70
Baranczewski P, Stanczak A, Sundberg K, Svensson R, Wallin A, Jansson J et al (2006) Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep 58:453–472
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
Michely JA, Meyer MR, Maurer HH (2018) Power of Orbitrap-based LC-high resolution-MS/MS for comprehensive drug testing in urine with or without conjugate cleavage or using dried urine spots after on-spot cleavage in comparison to established LC-MS(n) or GC–MS procedures. Drug Test Anal 10:158–163
Richter LHJ, Flockerzi V, Maurer HH, Meyer MR (2017) Pooled human liver preparations, HepaRG, or HepG2 cell lines for metabolism studies of new psychoactive substances? A study using MDMA, MDBD, butylone, MDPPP, MDPV, MDPB, 5-MAPB, and 5-API as examples. J Pharm Biomed Anal 143:32–42
Wagmann L, Richter LHJ, Kehl T, Wack F, Bergstrand MP, Brandt SD et al (2019) In vitro metabolic fate of nine LSD-based new psychoactive substances and their analytical detectability in different urinary screening procedures. Anal Bioanal Chem. https://doi.org/10.1007/s00216-018-1558-9
Schaefer N, Wojtyniak JG, Kettner M, Schlote J, Laschke MW, Ewald AH et al (2016) Pharmacokinetics of (synthetic) cannabinoids in pigs and their relevance for clinical and forensic toxicology. Toxicol Lett 253:7–16
Caspar AT, Westphal F, Meyer MR, Maurer HH (2018) LC-high resolution-MS/MS for identification of 69 metabolites of the new psychoactive substance 1-(4-ethylphenyl-)-N-[(2-methoxyphenyl)methyl] propane-2-amine (4-EA-NBOMe) in rat urine and human liver S9 incubates and comparison of its screening power with further MS techniques. Anal Bioanal Chem 410:897–912
Richter LHJ, Herrmann J, Andreas A, Park YM, Wagmann L, Flockerzi V et al (2019) Tools for studying the metabolism of new psychoactive substances for toxicological screening purposes—a comparative study using pooled human liver S9, HepaRG cells, and zebrafish larvae. Toxicol Lett 305:73–80
Sorensen LK (2011) Determination of cathinones and related ephedrines in forensic whole-blood samples by liquid-chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879:727–736
Wagmann L, Maurer HH (2018) Bioanalytical methods for new psychoactive substances. Handb Exp Pharmacol 252:413–439
Niessen WM, Correa RA (2016) Interpretation of MS–MS mass spectra of drugs and pesticides. Wiley, Hoboken
Linhart I, Himl M, Zidkova M, Balikova M, Lhotkova E, Palenicek T (2016) Metabolic profile of mephedrone: identification of nor-mephedrone conjugates with dicarboxylic acids as a new type of xenobiotic phase II metabolites. Toxicol Lett 240:114–121
Zidkova M, Linhart I, Balikova M, Himl M, Dvorackova V, Lhotkova E et al (2018) Identification of three new phase II metabolites of a designer drug methylone formed in rats by N-demethylation followed by conjugation with dicarboxylic acids. Xenobiotica 48:618–625
Pozo OJ, Ibanez M, Sancho JV, Lahoz-Beneytez J, Farre M, Papaseit E et al (2015) Mass spectrometric evaluation of mephedrone in vivo human metabolism: identification of phase I and phase II metabolites, including a novel succinyl conjugate. Drug Metab Dispos 43:248–257
Olesti E, Farre M, Papaseit E, Krotonoulas A, Pujadas M, de la Torre R et al (2017) Pharmacokinetics of mephedrone and its metabolites in human by LC-MS/MS. AAPS J 19:1767–1778
Uralets V, Rana S, Morgan S, Ross W (2014) Testing for designer stimulants: metabolic profiles of 16 synthetic cathinones excreted free in human urine. J Anal Toxicol 38:233–241
McNaney CA, Drexler DM, Hnatyshyn SY, Zvyaga TA, Knipe JO, Belcastro JV et al (2008) An automated liquid chromatography-mass spectrometry process to determine metabolic stability half-life and intrinsic clearance of drug candidates by substrate depletion. Assay Drug Dev Technol 6:121–129
Ellefsen KN, Wohlfarth A, Swortwood MJ, Diao X, Concheiro M, Huestis MA (2016) 4-Methoxy-alpha-PVP: in silico prediction, metabolic stability, and metabolite identification by human hepatocyte incubation and high-resolution mass spectrometry. Forensic Toxicol 34:61–75
Swortwood MJ, Ellefsen KN, Wohlfarth A, Diao X, Concheiro-Guisan M, Kronstrand R et al (2016) First metabolic profile of PV8, a novel synthetic cathinone, in human hepatocytes and urine by high-resolution mass spectrometry. Anal Bioanal Chem 408:4845–4856
Mardal M, Gracia-Lor E, Leibnitz S, Castiglioni S, Meyer MR (2016) Toxicokinetics of new psychoactive substances: plasma protein binding, metabolic stability, and human phase I metabolism of the synthetic cannabinoid WIN 55,212-2 studied using in vitro tools and LC-HR-MS/MS. Drug Test Anal 8:1039–1048
Gandhi AS, Wohlfarth A, Zhu M, Pang S, Castaneto M, Scheidweiler KB et al (2015) High-resolution mass spectrometric metabolite profiling of a novel synthetic designer drug, N-(adamantan-1-yl)-1-(5-fluoropentyl)-1H-indole-3-carboxamide (STS-135), using cryopreserved human hepatocytes and assessment of metabolic stability with human liver microsomes. Drug Test Anal 7:187–198
Acknowledgements
The authors would like to thank Daniel Arnold, Nora Toggweiler, and Armin A. Weber for their support and/or helpful discussion, and Thermo Fisher Scientific for providing a seed unit of the apparatus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The human material investigated was submitted to the authors’ laboratory for regular toxicological analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wagmann, L., Manier, S.K., Eckstein, N. et al. Toxicokinetic studies of the four new psychoactive substances 4-chloroethcathinone, N-ethylnorpentylone, N-ethylhexedrone, and 4-fluoro-alpha-pyrrolidinohexiophenone. Forensic Toxicol 38, 59–69 (2020). https://doi.org/10.1007/s11419-019-00487-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-019-00487-w