Abstract
Purpose
Exogenous Organic Matter (EOM) enriches and regenerates the soil, while solving the problem of landfilling waste such as sewage sludge and bottom sediments. The main purpose of the presented study was to estimate the influence of type and dosage of applied EOM on soil biological characteristics and supporting plant growth, with consideration of inoculation with PGPR (plant growth-promoting rhizobacteria).
Materials and methods
The soil samples were characterized by measuring key enzyme activities, determining the community-level physiological profiling (CLPP) using Biolog EcoPlates, abundance of microorganisms and evaluation physical and chemical properties.
Results and discussion
Application of EOM mostly increased enzyme activity as well as overall metabolic activity compared to control sample. Increasing the dose of poultry manure and sewage sludge from 20 to 40 t ha−1 increased dehydrogenase, acid and alkaline phosphatase activity. Moreover, the addition of EOM affected the metabolic activity and the number of selected groups of bacteria and fungi.
Conclusions
In the research it was proven that application of EOM leads to relatively rapid and positive changes in soil biological activity. The research also confirmed that the supporting factor for plant growth was the inoculation with PGPR bacteria. This approach, together with the reuse of organic wastes may become an attractive approach in sustainable cropping systems in a circular economy system.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Organic matter (OM) plays a key role in maintaining the physical, chemical and biological properties of soils, therefore its content is a major measure of a healthy soil (Raviv 2005; Leroy et al. 2008; Zhai et al. 2022). Its preservation and further accumulation are key to maintaining food security and mitigating climate change. Soil organic matter strongly affects biological activity, that is crucial for soil functioning, either directly (Raviv 2005) or indirectly by affecting soil structure and water retention (Fujino et al. 2008). Exogenous organic matter (EOM), among which we can mention manure or compost from organic waste, is used to supplement soil organic matter (SOM) (Leroy et al. 2008). EOM might also include residues of urban and industrial origin, as well as products of their processing (Marmo et al. 2004). Exogenous organic matter with potentially variable physicochemical properties can affect the soil ecosystem in various and sometimes unforeseen ways (Cayuela et al. 2009).
Biological activity is an important element of soil health, and microorganisms can play the role of bioindicators of the quality of the soil environment. Contrary to physical and chemical properties, which change slowly, biological properties are sensitive to even small fluctuations in environmental factors. All disturbances and changes in environmental ecosystems are reflected in changes in the diversity and metabolic activity of microorganisms, with changes in both their distribution and nutrient uptake (Margesin et al. 2014; Woźniak et al. 2022). Useful soil microorganisms have many important functions, such as the participation in transformation of nutrients, the breakdown of post-harvest residues, the stimulation of plant growth, soil bioremediation, and the improvement of the soil structure. Soil microbes and their functions are dynamic and complex and are therefore gaining more and more attention (Sofo and Ricciuti 2019; Candan et al. 2021). The use of a single biological method to assess soil conditions may not be representative, and it is therefore recommended that biological results are developed based on the group of parameters. One example is the use of the Biolog EcoPlates technique for the assessment of enzymatic activity and the abundance of various groups of soil microorganisms (Siebielec et al. 2020; Woźniak et al. 2022).
One of the most important parameters in studies on the soil ecosystem are enzymatic activities. Sources of soil enzymes include living and dead microorganisms, roots and residues, as well as soil organisms (Siebielec et al. 2020).
Soil enzyme activities are indicators of microbial functions and changes in soil biochemical processes. According to literature data, the activity of soil enzymes is a fast and sensitive indicator of soil environmental quality in the context of global climate changes as well as anthropogenic and natural factors (Liu et al. 2021). Recent decades have seen a rise in environmental awareness and an interest in more sustainable agricultural production systems, along with a circular economy. A safe alternative to agrochemicals is the use of PGPR (plant growth-promoting rhizobacteria), which has great potential to increase the productivity of agricultural crops exposed to environmental stress and nutrient deficiency, e.g., through the mobilisation of nutrients and the synthesis of various metabolites (phytohormones, siderophores) (Woźniak et al. 2019; Basu et al. 2021). Numerous studies have shown that microbial inoculants can induce changes in soil microorganisms and improve soil fertility, thus influencing soil quality and crop growth (Alori and Babalola 2018; Huang et al. 2022). Among other practices of sustainable agriculture is the recycling of EOM, which can be used as a soil amendment increasing soil fertility and yields and positively affects various soil functions. As a non-chemical fertiliser, EOM is necessary for organic agriculture to decrease agricultural dependencies on mineral fertilisers (Usowicz and Lipiec 2020; Moinard et al. 2021). According to several studies, EOM resources can be used to estimate their potential impact on crop productivity (Jia et al. 2018; Siebielec et al. 2018). However, the evaluation the potential use of EOM in agriculture requires additional studies taking into account various aspects to understand the processes accompanying EOM recycling. Information about the alteration of the biological activity and metabolic diversity of soil microorganisms in response to the addition of EOM and PSB inoculation is, however, limited (Siebielec et al. 2019).
Therefore, the aim of this study was to estimate the influence of the application of various types and dosage of EOM (poultry manure, bottom sediment and sewage sludge) on the activity of selected soil biological properties as well as on white mustard growth in a greenhouse pot study. Moreover, emphasis was placed on the interaction between EOM application and bacterial inoculation using PGPR strains.
2 Materials and methods
2.1 Experimental setup
The pot experiment was set up in 2022 in the summer period in the greenhouse of IUNG-PIB. Table 1 presents the range of EOM and bacterial strain combinations tested in the experiment. Three EOM additives (sewage sludge (S.S), bottom sediment (B.sed.) and poultry manure (M), were selected for this study.
The pot test was run for 16 weeks with three replicates for each combination. The contents of each pot (soil with the addition of EOM) were homogenised in plastic bags and transferred back to the pots. The pots were left for 3 weeks to allow the EOM supplements to react with the soil before sowing white mustard (Sinapis alba L.). Pots were watered with distilled water as needed.
Each pot was inoculated with the bacterial strains suspended in 10 mM MgSO4 during the germination period (approximately 3 weeks after sowing) with 100 mL of each bacterial suspension. The same amount of sterile 10 mM MgSO4 was added to the non-inoculated pots. Inoculation was repeated 3 weeks later, using the same procedure.
After 16 weeks of growth, the plants were harvested, dried and weighed to determine shoot dry weight. After harvest, the soil from each pot was thoroughly mixed so that the samples were homogeneous and sieved through a 2-mm sieve to remove residues including plant debris. Subsequently, soil aliquots were taken for further analysis. Each soil sample was divided into two parts. Fresh soil was used for microbiological analyses, and air-dried samples were used to determine chemical parameters.
2.2 Chemical soil analysis
Among the chemical parameters, soil pH was measured potentiometrically using a combined glass electrode in a 1:2 volume soil/deionised water slurry with a pH meter (edge multiparameter pHmeter, HANNA Instrument, Woonsocket, RI, USA). Available phosphorus (P) was determined by the Egner-Riehm colorimetric method after extraction with calcium lactate (0.02 M) in diluted HCl (0.01 M), followed by measurement in a Perkin Elmer Lambda 45 spectrometer, based on a colour reaction with ammonium molybdate. Available potassium (K) was measured after the same AAS calcium lactate extraction, using the AAnalyst 800 (Perkin Elmer, Waltham, MA, USA).
2.3 Microbiological soil analysis
2.3.1 Bacterial community-level physiological profiling (CLPP)
To monitor the metabolic profiles of all soil microbial communities, we used the Biolog EcoPlates (Biolog System Inc., Hayward, CA, USA). Analysis of the functional diversity was performed according to the manufacturer's instructions (Garland and Mills 1991). For Biolog data analysis, the AWCD value (average well-colour development), Shannon–Wiener index (H′), Shannon’s evenness index (E) and Richness value (R) were calculated to reflect the overall functional diversity of soil microbes (Frąc et al. 2012).
2.3.2 Soil enzyme activity
The determination of the activities of acid (AcP) and alkaline phosphatase (AlP) were measured with PNP (sodium p-nitrophenylphosphate), using the colorimetric method described by Tabatabai and Bremner (1969). Soil dehydrogenases activity (DHA) was determined by measuring the reduction of TTC (triphenyltetrazolium chloride) to triphenylformazan (TPF) according to the method of Casida et al. (1964). Each measurement was performed in triplicate.
2.3.3 Total abundance of cultivable bacteria and fungi
Determination of the total abundance of cultivable bacteria (Wallace and Lochhead 1950), Azotobacter spp. (Fenglerowa 1965), phosphorus-solubilising bacteria (PSB) (Pikovskaya 1948), copiotrophic and oligotrophic bacteria (Hattori and Hattori 1980), as well as the count of cultivable fungi (Martin 1950), were preformed using the soil-dilution method.
2.4 Bacterial strains
Bacterial inoculation of the plants in the greenhouse experiment was performed according to the procedures used in other studies (Siebielec et al. 2019; Balseiro-Romero et al. 2016; Becerra-Castro et al. 2013). Fresh cultures of bacterial strains (Strains 1 – genus of bacteria Streptomyces and Strains 2 – genus of bacteria Azotobacter) were grown in liquid media (Siebielec et al. 2021). Two strains of rhizobacteria that had previously been isolated from contaminated soils were used as inoculants. Streptomyces sp. (GenBank accession number MT658787) and Azotobacter sp. were isolated from a reclaimed slag pile (Siebielec et al. 2018, 2021). Strains 1 and 2 were isolated from the transect subjected to treatments with waste lime and sewage sludge. These strains exhibited phosphorus solubilisation capabilities in laboratory plate tests by creating a transparent halo zone around a colony grown on substrate rich in insoluble forms of phosphates. In a previous study, the Streptomyces strain showed a positive effect on the growth and yield of plants, with an increased plant availability of phosphorus on contaminated soils (Siebielec et al. 2021). Strains 1 and 2 were grown in a medium suitable for bacteria with the ability to solubilise phosphate (Pikovskaya 1948).
2.5 Plant biomass and chlorophyll index (SPAD)
After harvesting, the plants were dried, and the aboveground parts were separated from the roots; both aboveground parts and roots were weighed. In addition, the SPAD was determined to obtain additional characteristics during plant growth. The relative chlorophyll content (SPAD index) was measured with the SPAD 502 (Minolta, Tokyo, Japan) chlorophyll metre in four replications (Blackmer and Schepers 1994). Measurements were taken for 20 leaves in each pot and expressed as average values.
2.6 Statistical analysis
All experiments were performed in triplicate. All data are expressed as the mean ± standard deviation (SD). Statistical analyses were performed the Statistica 13.1 software (StatSoft, Inc., Tulsa, OK, USA). All results were analysed via post-hoc analysis using Tukey’s honestly significant difference (HSD) test at a significance level of p < 0.05. In addition, principal components analysis (PCA) was performed to determine the interaction of soil microbiological properties with poultry manure.
3 Results
3.1 The effect of EOM and soil inoculation on chemical soil properties
The initial pH of the soil was slightly neutral (pH 7.1; Table 2). Adding poultry manure resulted in a small increase in pH (pH 7.3). A similar pattern was observed for bottom sediment (pH 7.7; Table 3) and sewage sludge (pH 7.3; Table 4). However, only in case of bottom sediment, the difference was statistically significant. Inoculation had no significant effect on the pH value. Based on the results, the addition of some EOM types affected the availability of potassium (K) (Tables 2, 3 and 4). In the case of poultry manure, the lower rate did not increase K availability, but both strains increased this parameter significantly. At a greater dose, the addition of manure stimulated K availability as a result of providing a sufficient K pool. At a rate of 40 t ha, the strains did not further increase the K availability. The impact of bacterial strains on K availability to plants is an interesting and less explored issue. If the ability of the tested strains to solubilise K from K-rich manure is further confirmed, they can be considered as effective components of bioproducts, facilitating nutrient release. In soils treated with sewage sludge containing low K amounts, there was a decrease in K availability. This was likely due to intensive plant growth, which a substantial consumption of released K. This lower K availability at harvest does not necessarily mean that the strains were less effective. The P availability increased after adding the manure and the sewage sludge and after soil inoculation with both strains, although the differences were not statistically significant. When the bottom sediment was added to the soil, no significant differences were found in the levels of available forms of potassium and phosphorus (Table 3).
3.2 Microbiological soil analysis
3.2.1 Impact of treatments on enzyme activities
The additions of poultry manure and sewage sludge as single treatments significantly increased the activity of dehydrogenases (Tables 5, 6 and 7). The lowest dehydrogenase activity in the variant with chicken poultry manure was found in the control (16.18 TPF g d.m.−1 h−1), compared to the other variants, where the addition of EOM increased the enzymatic activity by 2.6–4.9 times. The highest value was recorded for the soil with the addition of poultry manure at a lower dose inoculated with bacterial Strain 2 (20M_Strain2—78.659 TPF g d.m.−1 h−1). This strain significantly accelerated the effect of manure on dehydrogenase activity. The greater manure dose improved dehydrogenases activity compared to the dose of 20 t ha−1, and at this level, the strains did not further enhance the measured numbers. No significant differences were observed for acid phosphatase activity in manure-treated soils. In contrast, the alkaline phosphatase activity after EOM supplementation and inoculation was highest (40M_Strain2—86.725 µg PNP g d.m.−1 h−1) in the combination with the higher dose of poultry manure inoculated with bacterial Strain 2. The lowest alkaline phosphatase value (38.659 µg PNP g d.m.−1 h−1) was recorded in the soil where no soil additive and bacterial strains were used (Table 5).
After the addition of bottom sediment and the inoculants used (Strains 1 and 2), the enzymatic activity of dehydrogenases and both phosphatases did not show significant differences in any of the variants used (Supplementary Table 1). The addition of sewage sludge stimulated the enzymatic activity of all soil enzymes tested, i.e., acid phosphatase, alkaline phosphatase and dehydrogenases. In the case of dehydrogenases and alkaline phosphatase, even the lower dose had a positive impact. The sludge effect on the enzymes was clearly rate-dependent. A significant increase in the activity of alkaline phosphatase was also observed after inoculation with Strain 1 accompanied with a greater dose of sewage sludge, as compared to sludge added alone, which resulted in a 27% increase (Table 6).
3.2.2 Impact of the treatments on the functional diversity of bacteria
The functional diversity and physiological profile of the microbial communities in the soil samples treated with EOM were assessed using phenotype microarray Biolog EcoPlates. For metabolic activity analysis, an incubation period of 120 h was selected because the slopes of the AWCD curves within this time showed optimal metabolic rates (Kong et al. 2013). In soil samples with added poultry manure, the AWCD indices reflecting the overall activity of the soil microorganisms did not show statistically significant differences. Likewise, no differences were found in the functional diversity indices (H', E and R; Table 7). However, in soil samples with the addition of bottom sediment (B.sed.), the microbial community in the soil treated with 20 t ha−1 of EOM (20B.sed_Strain1) showed the highest AWCD value. The control sample showed the lowest metabolic activity. Besides, no significant difference in other indices was observed in this experimental variant (Table 8). Sewage sludge amendment greatly increased the overall C use (AWCD), even as a single treatment (Table 9). The highest AWCD index values were detected for soil microbiota of sample 20S.S_Strain2 (2.122), followed by samples 40S.S_Strain2 (2.032) and 20S.S_N.I (1.965). The highest H′ values were found for the soil sample collected from the pot with the addition of 40 t ha−1 EOM and Strain 2 inoculation, indicating that the microorganisms exhibited a high metabolic diversity in these samples (Table 9). The Evenness index level decreased after sludge addition, indicating a less balanced C source use, which, however, fully recovered after inoculation – both strains at greater dose and on at lower EOM rate.
To visualise the Biolog EcoPlates results, the use patterns of the 31 carbon sources are presented as heatmap graphs for all soil samples from the pot experiment (Fig. 1). Based on the results, there was a slight difference in the carbon substrates metabolised by the soil microbiota of all samples for the different pot experiment variants. Generally, the microbial communities in all samples showed a similar trend in their use of the individual carbon sources. Overall, regardless of the type of EOM added, the most intensively used substrates were gamma-hydroxybutyric acid (except for the sample 20B.sed_N.I.), L-arginine and pyruvic acid methyl ester. In contrast, the lowest used carbon sources were 2-hydroxy benzoic acid and D,L-alpha-glycerol phosphate. By comparing the analysed samples, we observed that they differed mostly in relation to glycogen metabolism. The microbial community from soil samples 20S.S_Strain2 could more intensively use glycerol (Fig. 1). Importantly, soil inoculation with plant growth-promoting bacteria did not reduce the functional diversity of the soil microbiota.
3.2.3 Impact of treatments on total abundances of bacteria and fungi
Tables 10, 11 and 12 show the total abundances of cultivable bacteria and fungi. The results suggest a large variation in the total abundance after the application of EOM additives to the soil. Surprisingly, the abundance of bacteria of the genus Azotobacter was highest in combination with bottom sediments after the application of Strains 1 and 2. The lowest abundance of Azotobacter was recorded in combination with sewage sludge without inoculants. Apparently, sewage sludge addition suppressed the growth of Azotobacter to a certain extent, but it recovered with the application of Strains 1 and 2. The mechanism underlying the effect of Streptomyces is still unknown. Potentially, inoculation with Strain 1 altered the competition conditions among the microbial genera. The total number of PSB was significantly higher after the application of the additive and the inoculation with Strains 1 and 2 in all three cases. The counts of copiotrophic and oligotrophic bacteria were highest in combination with sewage sludge and poultry manure, both applied alone and in combination with inoculation. Copiotrophic and oligotrophic bacteria represent different strategies of survival in a very competitive soil environment—reproducing quickly in the presence of abundant nutrients (copiotrophs), or escaping to nutrient-poor niches (oligotrophs) (Stone et al. 2023). Analysis of the fungal abundances in soil samples showed that the introduction of EOM into the soil, as well as the inoculation with PGPR bacteria, contributed to a statistically significant increase in the total number of fungi. However, the underlying mechanisms are still unclear.
3.3 Principal component analysis
In the PCA on the abundances of groups of microorganisms, enzyme activities and plant biomass, the first two principal components (PCs) accounted for 52.27% and 19.94% of the total data variance, respectively (Fig. 2). The analysis allowed to clearly separate the soils based on the dose of poultry manure. The 40-t ha−1 dose was clustered at a long distance from the lower dose and the control. In addition, Strain 2 performed differently, based on its effect on bacteria of the genus Azotobacter and fungal abundance.
3.4 Plant growth and SPAD index
3.4.1 Plant biomass at harvest
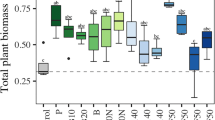
White mustard grown on soil with the addition of bottom sediment was characterised by pale green leaves with clear signs of chlorosis. This can be explained by the combined effect of nutrient deficiency and the addition of bottom sediment. In combination with bottom sediment, no positive effect of the inoculation with selected bacterial strains on the yield and development of white mustard was found. The applied variants of sewage sludge and poultry manure enabled a greater biomass production (Fig. 3). The total biomass of plants after poultry manure application at a dose of 20 t ha−1 was almost twice as high (20M_N.I.—7.65 g) as compared to that of the control soil (4.60 g). A positive effect of inoculation on plant yield was also demonstrated, which was most pronounced in the variant where poultry manure was applied at a dose of 40 t ha−1. A statistically significant positive effect compared to the control (40M_N.I.—15.20 g) was shown especially for strain 1 (40M_Strain1—18.10 g). Moreover, root growth was almost twice as high in the variant where poultry manure was applied to the soil at a dose of 20 t ha−1 and additionally inoculated with bacterial Strain 1 (20M_Strain1—4.83 g) (Table 13).
a Pot experiment (left side – EOM (poultry manure), right side – EOM (bottom sediment); b left side – no EOM, no inoculations; middle side –poultry manure, no inoculations; right side – poultry manure, inoculation with strains; c from the left: poultry manure, rate II, strain 1; poultry manure, rate II, strain 2; bottom sediment, rate II, strain 2; sewage sludge, rate II, strain 2; sewage sludge, rate I, strains 1; sewage sludge, rate II, strain 1
The yield of plants in bottom sediment-treated soil did not show significant differences in terms of root and aboveground biomass. Similarly, no significant differences were observed with additional bacterial inoculation. Also, the dose of 40 t ha−1 of bottom sediment applied to the soil and additionally inoculated with Strain 2 produced the lowest total plant biomass (40B.sed_Strain2—2.823 g) among all tested variants (Table 14).
After the application of sewage sludge to the soil, the yields of plants were significantly higher than in case of other EOMs tested. In addition, significant differences were noted in the yield of aboveground parts in the combination where sewage sludge was applied at a dose of 40 t ha−1 and inoculation with Strain 1 (40S.S_Strain1—10.38 g) compared to uninoculated soil (40S.S_N.I.—7.52 g). A similar relationship was observed for total biomass (40S.S_Strain1—18.21 g vs. 40S.S_N.I.—16.77 g). Interestingly, Strain 2 caused a decrease in root development, especially at a greater rate of EOM. Apparently, the strain enhanced soil nutrient availability, and therefore, the plants were not forced to excessively develop their root system (Table 15).
3.4.2 Chlorophyll content (SPAD index) of plants with EOM addition
The chlorophyll content is an important parameter for measuring the condition of plants. In this study, the chlorophyll index was not measured in plants grown in bottom sediment-amended soils since the leaves showed excessive chlorosis. No statistically significant differences were found in the plants growing on soil with manure added in any of the combinations used. Nevertheless, the plants growing in the control soil showed the lowest chlorophyll index value (Table 16). In contrast, the test plants growing in soil with the addition of sewage sludge showed a higher chlorophyll contents, irrespective of the variant, as compared to the control. The addition of Strain 2 resulted in the highest index for the soil treated with sewage sludge at a dose of 40 t ha−1 (Table 17).
4 Discussion
Research on the use of EOM (e.g., bottom sediment, sewage sludge) to agricultural soils has mainly focused on effects on plants and the fate of heavy metals in soil (Kazberuk et al. 2021). There is limited information as how EOM can influence soil microbiological and biochemical properties with regard to maintaining soil quality and productivity. Therefore, we investigated the impacts of various types and dosage of EOM on plant growth and soil microbial characteristics.
4.1 Chemical soil analysis
With the addition of bottom sediment to the soil, we observed an increase in soil pH from 6.5 to above 7.2.There is evidence that fertiliser additives in the form of EOM can affect the soil pH. In a previous study, the fresh cattle poultry manure immediately raised soil pH and nutrient availability in the Peace River region of Alberta, Canada.. The higher pH in manure-modified soils was primarily attributed to the buffering of bi-carbonate and organic acids present in cattle poultry manure (Whalen et al. 2000). In other stusies the application of liquid sludge to the soil environment raised the soil pH from 7.3 to 7.7 (Achkir et al. 2022). Similar results were obtained by other researchers, who noted an increase in soil pH after applying sewage sludge to the soil (Zhang et al. 2004).
Concerning the effect of inoculation, in the present study addition of bacterial inoculum had no significant effect on the pH values of the soil treated with the addition of organic matter., This is consistent with our previous study in which neither soil inoculation nor plant species had an effect on the soil pH (Siebielec et al. 2019).
In the present study the manure and sewage sludge altered K and P plant availability. According to literature data, the use of poultry manure has physical and chemical effects on soil properties. In addition, the use of poultry manure may increase the availability of P. The enhanced P availability and soil organic carbon content might result in increased yields. Some studies show that poultry manure can increase the level of dissolved P in the soil by reducing the adsorption power of P in the soil, resulting in higher P losses compared to the use of chemical fertilisers (Lu et al. 2021; Shepherd and Withers 1999). In another study, soils enriched with a dose of 40 g of kg−1 poultry manure were characterised by an almost four-fold increase in the available P and K forms for plants compared to unmodified soils (Whalen et al. 2000). Nevertheless, it should be noted that such effects are specific to the type of organic fertiliser used, the total doses of inorganic fertilisers and the prevailing soil conditions (Lu et al. 2021; Wang et al. 2019). Similarly, in our study the effect of EOM on nutrient availability was EOM-specific.
4.2 Microbiological soil analysis
Soil enzymes are the main mediators and catalysts of many important soil processes, such as organic matter decomposition and the release of substances necessary for plant growth. Enzymatic activities, which can be expressed by dehydrogenase or acid and alkaline phosphatase rates, are major biochemical indicators used to describe the biological quality of agricultural soils (Burns et al. 2013). In our study, the addition of EOM and the inoculation with selected bacterial strains resulted in the stimulation of alkaline phosphatase and dehydrogenases. Similar relationships were obtained in previous studies conducted on soils with the addition of compost and inoculation with bacterial strains. This approach, tested for contaminated soils, resulted in stimulation of plant growth and soil biological activity (Siebielec et al. 2021). Combined effects of EOM and bacterial strains have been tested rarely, however more information exists on the effects of individual EOM application.. Afangide et al. (2021) confirmed the effectiveness of poultry and pig fertilisers in improving soil properties in a long-term (33-year) field experiment, including substantial increase in the dehydrogenase effectiveness. Several other studies also confirmed the effect of EOM application on the parameters related to the enzymatic activity of the tested soils (Lee et al. 2004; Siebielec et al. 2018, 2019, 2021; Das et al. 2017; Francioli et al. 2016). The Biolog System has been used by many authors for the monitoring of the functional status of microorganisms in the soil under the influence of various factors (Woźniak et al. 2022; Siebielec et al. 2020). Our study demonstrated that all tested EOM types influenced the functional activity of the soil microbial communities. Probably, the increase in microbial activity is related to the introduction of nutrients and energy through the addition of EOM, with a wide range of substrates. According to Gryta et al. (2020), a high nutrient content stimulates microbial metabolism (Gryta et al. 2020). These authors obtained similar results, showing that the addition of EOM to soils can change the functional diversity of soil microorganisms; however, the degree of modification depends on the type of additive used. Also, Fließbach and Mäder (1997) showed that supplementation with poultry manure compost resulted in higher diversity indices, possibly as a result of the growth of additional microorganisms after OM incorporation. In order to assess the diversity and total number of bacteria, classical plate methods are still used to quantify the total abundances of bacteria and fungi or, alternatively, the abundance of particular bacterial groups responsible for certain microbially driven processes (Siebielec et al. 2018; 2021). Literature data prove that bacteria of the genus Azotobacter have the ability to fix atmospheric nitrogen (Martyniuk 2008) and are sensitive to changes in soil properties, including soil pH. Therefore, these free-living diazotrophs are highly important for fully efficient soil ecosystems. In our study, the use of EOM had a positive effect on Azotobacter populations, which in turn affects the growth and development of plants through nitrogen mobilization. The higher number of bacteria of the genus Azotobacter in Strain 1-treated soil was clearly a result of introducing Azotobacter as the inoculant. Importantly, after harvesting, the count of Azotobacter was still greater than that in the control soil, despite strong competition. The mechanism of Azotobacter growth stimulation by Strain 2 is still unclear, and further studies on the relationships between these genera are needed. Hypothetically, Streptomyces can stimulate Azotobacter growth through nutrient mobilisation or combating opponents of Azotobacter.
The presence of PSB plays a key role in the transformation and biogeochemical cycling of P in the soil environment (Yu et al. 2019). Our data show that the introduction of EOM, both separately and in combination with inoculation, contributed to an increase in the total number of PSB in relation to the control soil. It can be assumed that the strong growth of bacteria in the tested combinations was associated with the absorption of nutrients by microorganisms from EOM additives. In addition to bacteria, fungi play a fundamental role in the circulation of nutrients in the soil and in energy flow. Zhu et al. (2019) reported that the presence of some fungal populations may increase plant stress tolerance. It is therefore likely that the large number of fungi in our pot experiment played a significant role in stabilising plant development after the addition of poultry manure, bottom sediment and sewage sludge, as shown in the Tables 10, 11 and 12. Similarly, Siebielec et al. (2018) observed that compost addition increased the abundances of PSB and fungi.
4.3 Plant growth and SPAD index
In our short-term experiment, we showed that both the addition of exogenous matter alone as well as combined with bacterial inoculation influenced the yield of the test plant. Literature data confirm the positive impact of sewage sludge on crops, including cereals, vegetable and horticultural plants, and pastures (Pascual et al. 2004; Antolín et al. 2005).
Our results indicate that the use of sewage sludge increases crop yields and improves the photosynthetic activities of the test plant. Among the tested combinations, the best results were obtained after the use of sewage sludge combined with microbial inoculants. Similar relationships were observed when assessing the soil microbiological and crop responses in other studies (Panasiewicz et al. 2019), confirming our hypothesis that the combination of EOM addition and soil inoculation has a multilateral beneficial effect.
Studies on the combined effect of EOM and soil inoculation on physiological status of crops are scarce. However, other authors also reported that the addition of sewage sludge had a positive effect on plant growth, as indicated by the production of pods and seeds (Singh and Agrawal 2010). Chitdeshwari et al. (2002) determined the effects of EOM supplements on corn and bean yields under greenhouse conditions; sewage sludge stimulated the yield of all plants compared to the unmodified control in a dose-dependent manner. Tsakou et al. (2001) reported a positive effect of sewage sludge on the yield and quality (fibres and seeds) of cotton; the increase in productivity was attributed to an increased nutrient availability. The macronutrients contained in sewage sludge are a good source of nutrients for plants, and the organic compounds improve the soil properties (Singh and Agrawal 2008).
When testing plant responses to fertilisation regimes, particular attention should be paid to the nutritional status of the plant and its performance. Certain soil amendments might result in nutrient deficiencies, potentially leading to a reduced productivity. One of the rapid tools to assess the nutritional status of growing plants is the chlorophyll metre, which enables quick and simple measurements of the chlorophyll content in plant leaves (Zandonadi et al. 2016). In our experiment, such measurements clearly confirmed that the use of EOM (poultry manure and sewage sludge) had a positive effect on the chlorophyll content. In addition, a positive effect on the chlorophyll content was noted in a variant with sewage sludge combined with Strain 2, which improved the nutritional status of the plant.
5 Conclusions
This study confirms that soil amendment with poultry manure and sewage sludge can stimulate plant growth and improve some soil parameters. This approach also stimulates the enzymatic and metabolic activity of soil and the functionally important groups of bacteria, such as nitrogen-fixing, copiotrophic, oligotrophic and P-solubilising bacteria. Bottom sediment addition had no positive effect on plant yield.
Inoculation with bacterial strains might further improve the effect of EOM additives. Of particular importance in this study was Strain 1, which showed a tendency to activate plant growth after EOM application. Moreover, it had a positive effect on the microbiological soil parameters, suggesting that it is a valuable component of biofertilisers, in line with the implementation of sustainable agriculture and nature-based solutions. This also suggests that there is a need for further research on the potential of bacteria interacting with exogenous organic matter.
We have not observed any negative effects of soil inoculation on soil microbiome characterized by functional diversity, abundance and activity. This puts some new light on competition between bacteria introduced to soil and native bacterial communities. It was also proved that abundance of Azotobacter applied to soil as the inoculation suspension remains through a period of 3 months despite the competition with native strains.
Data availability
The data used for the analyses presented in this work is available from the corresponding author on reasonable request.
References
Achkir A, Aouragh A, El Mahi M, El Lotfi EM, Labjar N, Badza T, El Moussaoui T (2022) Effects of different rates of liquid sewage sludge amendment on nutrient content of the soil in Rabat. Morocco Environ Sci Proc 16:19. https://doi.org/10.3390/environsciproc2022016019
Afangide AI, Egboka NT, Inyang P, Tom CT, Ebele CD (2021) Influence of animal manures on dehydrogenase activities and microbial population. In: Ahmed H, Dabwan A (eds) Tropical Soil of Southeastern Nigeria. Zibeline International Publishing, INWASCON Technology Magazine (iTECH MAG), pp 50–54
Alori ET, Babalola OO (2018) Microbial inoculants for improving crop quality and human health in Africa. Front Microbiol 9:2213. https://doi.org/10.3389/fmicb.2018.02213
Antolín MC, Pascual I, García C, Polo A, Sánchez-Díaz M (2005) Growth, yield and solute content of barley in soils treated with sewage sludge under semiarid Mediterranean conditions. Field Crops Res 94:224–237. https://doi.org/10.1016/j.fcr.2005.01.009
Balseiro-Romero M, Gkorezis P, Kidd PS, Vangronsveld J, Monterroso C (2016) Enhanced degradation of diesel in the rhizosphere of Lupinus luteus after inoculation with diesel-degrading and PGP bacterial strains. J Environ Qual 45:924–932. https://doi.org/10.2134/jeq2015.09.0465
Basu A, Prasad P, Das SN, Kalam S, Sayyed RZ, Reddy MS, El Enshasy H (2021) Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 13:1140. https://doi.org/10.3390/su13031140
Becerra-Castro C, Prieto-Fernández Á, Kidd PS, Weyens N, Rodríguez-Garrido B, Touceda-González M, Acea MJ, Vangronsveld J (2013) Improving performance of Cytisus striatus on substrates contaminated with hexachlorocyclohexane (HCH) isomers using bacterial inoculants: Developing a phytoremediation strategy. Plant Soil 362:247–260. https://doi.org/10.1007/s11104-012-1276-6
Blackmer TM, Schepers JS (1994) Techniques for monitoring crop nitrogen status in corn. Commun Soil Sci Plant Anal 25:1791–1800. https://doi.org/10.1080/00103629409369153
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol Biochem 58:216–234. https://doi.org/10.1016/j.soilbio.2012.11.009
Candan ED, İdil N, Candan O (2021) The microbial community in a green turtle nesting beach in the Mediterranean: application of the Biolog EcoPlate approach for beach pollution. Environ Sci Pollut Res 28(36):49685–49696. https://doi.org/10.1007/s11356-021-14196-8
Casida LE, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Cayuela ML, Sinicco T, Mondini C (2009) Mineralization dynamics and biochemical properties during initial decomposition of plant and animal residues in soil. Appl Soil Ecol 4:118–127. https://doi.org/10.1016/j.apsoil.2008.10.001
Chitdeshwari T, Savithri P, Mahimairaj S (2002) Effect of sewage biosolid composts on the yield of crops Indian. J Environ Protect 21:10
Das S, Jeong ST, Das S, Kim PJ (2017) Composted cattle manure increases microbial activity and soil fertility more than composted swine manure in a submerged rice paddy. Front Microbiol 5(8):1702. https://doi.org/10.3389/fmicb.2017.01702
Fenglerowa W (1965) Simple method for counting Azotobacter in soil samples. Acta Microbiol Pol 14:203–206
Fließbach A, Mäder P (1997) Carbon source utilization by microbial communities in soils under organic and conventional farming practice. In: Insam H, Rangger A (eds) Microbial Communities: Functional Versus Structural Approaches. Springer-Verlag, Berlin, Heidelberg, pp 37–48
Frąc M, Oszust K, Lipiec J (2012) Community level physiological profiles (CLPP), characterization and microbial activity of soil amended with dairy sewage sludge. Sensors 12:3253–3268. https://doi.org/10.3390/s120303253
Francioli D, Schulz E, Lentendu G, Wubet T, Buscot F, Reitz T (2016) Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front Microbiol 7:1446. https://doi.org/10.3389/fmicb.2016.01446
Fujino C, Wada S, Konoike T, Toyota K, Suga Y, Ikeda J (2008) Effect of different organic amendments on the resistance and resilience of the organic matter decomposing ability of soil and the role of aggregated soil structure. Soil Sci Plant Nutr 54:534–542. https://doi.org/10.1111/j.1747-0765.2008.00281.x
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57(8):2351–2359. https://doi.org/10.1128/aem.57.8.2351-2359.1991
Gryta A, Frąc M, Oszust K (2020) Genetic and metabolic diversity of soil microbiome in response to exogenous organic matter amendments. Agronomy 10:546. https://doi.org/10.3390/agronomy10040546
Hattori R, Hattori T (1980) Sensitivity to salts and organic compounds of soil bacteria isolated on diluted media. J Gen Appl Microbiol 26:1–14. https://doi.org/10.2323/jgam.26.1
Huang Z, Ruan S, Sun Y, Cheng X, Dai J, Gui P, Yu M, Zhong Z, Wu J (2022) Bacterial inoculants improved the growth and nitrogen use efficiency of Pyrus betulifolia under nitrogen-limited conditions by affecting the native soil bacterial communities. Appl Soil Ecol 491:104285. https://doi.org/10.1016/j.apsoil.2021.104285
Jia W, Qin W, Zhang Q, Wang X, Ma Y, Chen Q (2018) Evaluation of crop residues and manure production and their geographical distribution in China. J Clean Prod 188:954–965. https://doi.org/10.1016/j.jclepro.2018.03.300
Kazberuk W, Szulc W, Rutkowska B (2021) Use bottom sediment to agriculture—Effect on plant and heavy metal content in soil. Agronomy 11:1077. https://doi.org/10.3390/agronomy11061077
Kong X, Wang C, Ji M (2013) Analysis of microbial metabolic characteristics in mesophilic and thermophilic biofilters using Biolog plate technique. Chem Eng J 230:415–421. https://doi.org/10.1016/j.cej.2013.06.073
Lee JJ, Park RD, Kim YW, Shim JH, Chae DH, Rim YS, Sohn BK, Kim TH, Kim KY (2004) Effect of food waste compost on microbial population, soil enzyme activity and lettuce growth. Biores Technol 93:21–28. https://doi.org/10.1016/j.biortech.2003.10.009
Leroy BLM, Herath S, Sleutel S, De Neve S (2008) The quality of exogenous organic matter: Short-term effects on soil physical properties and soil organic matter fractions. Soil Use and Manage 24(2):139–147. https://doi.org/10.1111/j.1475-2743.2008.00142.x
Liu C, Song Y, Dong X, Wang X, Ma X, Zhao G, Zang S (2021) Soil enzyme activities and their relationships with soil C, N, and P in peatlands from different types of permafrost regions, Northeast China. Front Environ Sci 9:6707. https://doi.org/10.3389/fenvs.2021.670769
Lu Y, Gao Y, Nie J, Liao Y, Zhu Q (2021) Substituting chemical P fertilizer with organic manure: effects on double-rice yield, phosphorus use efficiency and balance in subtropical China. Sci Rep 11:8629. https://doi.org/10.1038/s41598-021-87851-2
Margesin R, Minerbi S, Schinner F (2014) Long-term monitoring of soil microbiological activities in two forest sites in South Tyrol in the Italian Alps. Microbes Environ 29:277–285. https://doi.org/10.1264/jsme2.ME14050
Marmo L, Feix I, Bourmeau E, Amlinger F, Bannick CG, De Neve S, Favoino E, Gendebien A, Gibert J, Givelet M, Leifert I, Morris R, Rodriguez Cruz A, Ruck F, Siebert S, Tittarelli F (2004) Reports of the technical working groups established under the thematic strategy for soil protection. Volume - III. Organic matter and biodiversity. Taskgroup 4 onexogenous organic matter. Office for Official Publications of the European Communities, Luxembourg. http://ec.europa.eu/environment/soil/pdf/vol3.pdf. Accessed 28 Nov 2022
Martin JP (1950) Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci 69:215–232
Martyniuk S (2008) Znaczenie procesu biologicznego wiązania azotu atmosferycznego w rolnictwie ekologicznym. J Res Appl Agric Eng 53:9–14
Moinard V, Levavasseur F, Houot S (2021) Current and potential recycling of exogenous organic matter as fertilizers and amendments in a French peri-urban territory. Resour Conserv Recycl 169:105523. https://doi.org/10.1016/j.resconrec.2021.105523
Panasiewicz K, Niewiadomska A, Sulewska H, Wolna-Maruwka A, Borowiak K, Budka A, Ratajczak K (2019) The effect of sewage sludge and BAF inoculant on plant condition and yield as well as biochemical and microbial activity of soil in willow (Salix viminalis L.) culture as an energy crop. Peer J 11(7):e6434. https://doi.org/10.7717/peerj.6434
Pascual I, Antolín MC, García C, Polo A, Sánchez-Díaz M (2004) Plant availability of heavy metals in a soil amended with a high dose of sewage sludge under drought conditions. Biol Fertil Soils 40(5):291–299. https://doi.org/10.1007/s00374-004-0763-1
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiology 17:362–370
Raviv M (2005) Production of high-quality composts for horticultural purposes: a mini-review. HortTechnology 15:52–57. https://doi.org/10.21273/HORTTECH.15.1.0052
Shepherd MA, Withers PJ (1999) Applications of poultry litter and triple superphosphate fertilizer to a sandy soil: Effects on soil phosphorus status and profile distribution. Nutr Cycl Agroecosyst 54:233–242. https://doi.org/10.1023/A:1009744706679
Siebielec S, Siebielec G, Marzec-Grządziel A, Pecio M, Stuczyński T (2021) Testing combined effect of amendments and inoculation with bacteria for improving phytostabilisation of smelter waste extremely contaminated with trace elements. Agronomy 11(10):2064. https://doi.org/10.3390/agronomy11102064
Siebielec S, Siebielec G, Stuczynski T, Sugier P, Grzeda E, Grzadziel J (2018) Long term insight into biodiversity of a smelter wasteland reclaimed with biosolids and by-product lime. Sci Total Environ 636:1048–1057. https://doi.org/10.1016/j.scitotenv.2018.04.372
Siebielec S, Siebielec G, Sugier P, Woźniak M, Grzadziel J, Gałązka A, Stuczyński T (2020) Activity and diversity of microorganisms in root zone of plant species spontaneously inhabiting smelter waste piles. Molecules 25:5638. https://doi.org/10.3390/molecules25235638
Siebielec S, Siebielec G, Urbaniak M, Smreczak B, Grzęda E, Wyrwicka A, Kidd P (2019) Impact of rhizobacterial inoculants on plant growth and enzyme activities in soil treated with contaminated bottom sediments. Int J Phyto 21:325–333. https://doi.org/10.1080/15226514.2018.1524833
Singh RP, Agrawal M (2008) Potential benefits and risks of land application of sewage sludge. Waste Manage 28(2):347–358. https://doi.org/10.1016/j.wasman.2006.12.010
Singh RP, Agrawal M (2010) Effect of different sewage sludge applications on growth and yield of Vigna radiata L. field crop: Metal uptake by plant. Ecol Eng 36(7):969–972. https://doi.org/10.1016/j.ecoleng.2010.03.008
Sofo A, Ricciuti P (2019) A standardized method for estimating the functional diversity of soil bacterial community by Biolog® EcoPlatesTM Assay—The case study of a sustainable olive orchard. Appl Sci 9:4035. https://doi.org/10.3390/app9194035
Stone BW, Dijkstra P, Finley BK, Fitzpatrick R, Foley MM, Hayer M, Hungate BA (2023) Life history strategies among soil bacteria—dichotomy for few, continuum for many. ISME J 17(4):611–619. https://doi.org/10.1038/s41396-022-01354-0
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307. https://doi.org/10.1016/0038-0717(69)90012-1
Tsakou A, Roulia M, Christodoulakis NS (2001) Growth of cotton plants (Gossypium hirsutum) as affected by water and sludge from a sewage treatment plant: II. Seed and fiber yield and heavy metal accumulation. Bull Environ Contam Toxicol 66(6):735–742
Usowicz B, Lipiec J (2020) The effect of exogenous organic matter on the thermal properties of tilled soils in Poland and the Czech Republic. J Soils Sediments 20(1):365–379. https://doi.org/10.1007/s11368-019-02388-2
Wallace RH, Lochhead AG (1950) Qualitative studies of soil microorganisms: IX. Amino acid requirements of rhizosphere bacteria. Can J Res 28:1–6
Wang H, Xu J, Liu X, Zhang D, Li L, Sheng L (2019) Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Tillage Res 195:104382. https://doi.org/10.1016/j.still.2019.104382
Whalen JK, Chang C, Clayton GW, Carefoot JP (2000) Cattle manure amendments can increase the pH of acid soils. Soil Sci Soc Am J 64(3):962–966. https://doi.org/10.2136/sssaj2000.643962x
Woźniak M, Gałązka A, Siebielec G, Frąc M (2022) Can the biological activity of abandoned soils be changed by the growth of Paulownia elongata × Paulownia fortunei?-Preliminary study on a young tree plantation. Agriculture 12:128. https://doi.org/10.3390/agriculture12020128
Woźniak M, Gałązka A, Tyśkiewicz R, Jaroszuk-Ściseł J (2019) Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the Biolog GEN III MicroPlateTM test. Int J Mol Sci 20:5283. https://doi.org/10.3390/ijms20215283
Yu LY, Huang HB, Wang XH, Li S, Feng NX, Zhao HM (2019) Novel phosphate-solubilising bacteria isolated from sewage sludge and the mechanism of phosphate solubilisation. Sci Total Environ 658:474–484. https://doi.org/10.1016/j.scitotenv.2018.12.166
Zandonadi CHS, Albuquerque CJB, de Freitas RS (2016) Chlorophyll index (SPAD) and macronutrients relation and productive performance of sorghum hybrids in different sowing dates. Aust. J Crop Sci 10(4):546–555. https://doi.org/10.21475/ajcs.2016.10.04.p7354x
Zhai X, Zhang L, Wu R, Wang M, Lian J, Munir MAM, Chen D, Liu L, Yang X (2022) Molecular composition of soil organic matter (SOM) regulate qualities of tobacco leaves. Sci Rep 12:15317. https://doi.org/10.1038/s41598-022-19428-6
Zhang S, Wang S, Shan X, Mu H (2004) Influences of lignin from paper mill sludge on soil properties and metal accumulation in wheat. Biol Fertil Soils 40:237–242. https://doi.org/10.1007/s00374-004-0771-1
Zhu LY, Wang XH, Chen FF, Li CH, Wu LC (2019) Effects of the successive planting of Eucalyptus urophylla on soil bacterial and fungal community structure, diversity, microbial biomass, and enzyme activity. Land Degrad Dev 30:636–646. https://doi.org/10.1002/ldr.3249
Acknowledgements
The authors greatly acknowledge Emilia Grzęda for help in managing the greenhouse study and in conducting the enzyme measurements.
Funding
The work was carried out as part of the statutory topic 2.41/1.03 IUNG-PIB.
Author information
Authors and Affiliations
Contributions
Małgorzata M. Woźniak: Conceptualisation, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Roles/Writing—original draft, Writing – review & editing, Visualisation, Project administration. Sylwia Siebielec: Conceptualisation, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Roles/Writing – original draft, Writing – review & editing, Visualisation, Project administration. Grzegorz Siebielec: Conceptualisation, Methodology, Formal analysis, Investigation, Resources, Roles/Writing—original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition. Jolanta Bojarszczuk: Methodology, Data curation. Anna Gałązka: Resources. Magdalena Urbaniak: Resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible editor: Zucong Cai
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Woźniak, M.M., Siebielec, S., Siebielec, G. et al. Microbially modified effect of exogenous organic matter on soil chemical and biological indices and plant responses. J Soils Sediments 24, 70–85 (2024). https://doi.org/10.1007/s11368-023-03632-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03632-6