Abstract
Purpose
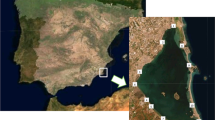
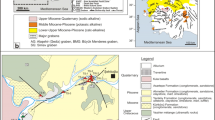
The geochemistry of pore waters from sediments deposited in the semi-enclosed bay of the Zrmanja River estuary, encompassing two small interconnected basins, the Novigrad Sea and the Karin Sea, was investigated. The conducted research aimed to identify diagenetic processes occurring in surficial bottom sediments and to assess the impact of these reactions on trace element concentrations in the overlying water.
Materials and methods
Sediment pore waters were extracted from sediment cores in a nitrogen atmosphere. Multielemental analysis of prepared samples was performed by high-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS). All samples were analysed for the total concentration of 15 elements (As, Ba, Co, Cr, Fe, Li, Mn, Mo, Ni, Rb, Sb, Sr, Ti, U and V).
Results and discussion
Results indicate enrichment of sediment pore waters in the majority of measured elements relative to the estuary water. Degradation of organic matter and dissolution of Mn-Fe-oxyhydroxides under suboxic and anoxic conditions were identified as principal sources of dissolved cations in the studied pore waters. While sediments from the Novigrad Sea act as a permanent geochemical sink, the estimated fluxes for the Karin Sea sediments indicate transfer of certain elements from the sediment back to the water column, amounting to ~ 0.3 μg m−2 day−1 for Co, Ti and U; ~ 0.7 μg m−2 day−1 for As; between 1 and 5 μg m−2 day−1 for Mo, Ni and Ba; and ~ 70 μg m−2 day−1 for Fe and Mn.
Conclusions
In the Zrmanja River estuary, the observed differences in the sediment pore water geochemistry and the role of the sediments in terms of mass exchange at the sediment-water interface could not be solely attributed to the sediment particle characteristics, but are considered to be a combined effect of organic matter input and local hydrogeological setting.







Similar content being viewed by others
References
Baldi F, Pepi M, Burrini D, Kniewald G, Scali D, Lanciotti E (1996) Dissolution of barium from barite in sewage sludges and cultures of Desulfovibrio desulfuricans. Appl Environ Microbiol 62:2398–2404
Balistrieri LS, Murray JW, Paul B (1994) The geochemical cycling of trace elements in a biogenic meromictic lake. Geochim Cosmochim Acta 58:3993–4008
Barnes CE, Cochran JK (1993) Uranium geochemistry in estuarine sediments: controls on removal and release processes. Geochim Cosmochim Acta 57(3):555–569
Beck M, Dellwig O, Schnetger B, Brumsack H-J (2008) Cycling of trace metals (Mn, Fe, Mo, U, V, Cr) in deep pore waters of intertidal flat sediments. Geochim Cosmochim Acta 72:2822–2840
Berner RA (1980) Early diagenesis: a theoretical approach. Princeton University Press, Princeton
Burić Z, Cetinić I, Viličić D, Caput Mihalić K, Carić M, Olujić G (2007) Spatial and temporal distribution of phytoplankton in a highly stratified estuary (Zrmanja, Adriatic Sea). Mar Ecol 28:169–177
Chow TJ, Goldberg ED (1960) On the marine chemistry of barium. Geochim Cosmochim Acta 20:192–198
Christensen JP, Devol AH, Smethie WM (1984) Biological enhancement of solute exchange between sediments and bottom water on the Washington continental shelf. Cont Shelf Res 3:9–23
De Lange GJ (1986) Chemical composition of interstitial water in cores from the nares abyssal plain (Western North Atlantic). Oceanol Acta 9:159–168
Di Toro DM, Mahony JD, Hansen DJ, Scott KJ, Hicks MB, Mayer SM (1990) Toxicity of cadmium in sediments: the role of acid volatile sulfide. Environ Toxicol Chem 9:1487–1502
Fiket Ž, Roje V, Mikac N, Kniewald G (2007) Determination of arsenic and other trace elements in bottled waters by high resolution inductively coupled plasma mass spectrometry. Croat Chem Acta 80:91–100
Fiket Ž, Mikac N, Kniewald G (2017a) Sedimentary records of the Zrmanja River estuary, eastern Adriatic coast—natural vs. anthropogenic impacts. J Soils Sediments 17(7):1905–1916
Fiket Ž, Pikelj K, Ivanić M, Barišić D, Vdović N, Dautović J, Žigovečki Gobac Ž, Mikac N, Bermanec V, Sondi I, Kniewald G (2017b) Origin and composition of sediments in a highly stratified karstic estuary: an example of the Zrmanja River estuary (eastern Adriatic, Croatia). Reg Stud Mar Sci 16:67–78. https://doi.org/10.1016/j.rsma.2017.08.001
Fiket Ž, Ivanić M, Furdek Turk M, Mikac N, Kniewald G (2018) Distribution of trace elements in waters of the Zrmanja River estuary (eastern Adriatic coast, Croatia). Croat Chem Acta 91(1):29–41
Friedrich J, Janssen F, Aleynik D, Bange HW, Boltacheva N, Çagatay MN, Dale AW, Etiope G, Erdem Z, Geraga M, Gilli A, Gomoiu MT, Hall POJ, Hansson D, He Y, Holtappels M, Kirf MK, Kononets M, Konovalov S, Lichtschlag A, Livingstone DM, Marinaro G, Mazlumyan S, Naeher S, North RP, Papatheodorou G, Pfannkuche O, Prien R, Rehder G, Schubert CJ, Soltwedel T, Sommer S, Stahl H, Stanev EV, Teaca A, Tengberg A, Waldmann C, Wehrli B, Wenzhöfer F (2014) Investigating hypoxia in aquatic environments: diverse approaches to addressing a complex phenomenon. Biogeosciences 11(4):1215–1259
Goldberg E, Arrhenius G (1958) Chemistry of pelagic sediments. Geochim Cosmochim Acta 13:153–212
Google Earth (2018) Town Obrovac, Croatia 44◦12′36′′ N, 15◦42′00′′ W, viewed 20 May 2018
Grimani I, Šikić K, Šimunić A (1975a) Basic Geological Map of SFRY 1:100 000, Sheet Knin L33–141 [in Croatian], Institute of Geology, Zagreb (1961-1969): Federal Geological Institute, Beograd
Grimani I, Juriša M, Šikić K, Šimunić A (1975b) Basic Geological Map SFRY 1:100 000. Geology of the sheet Knin, [in Croatian], Institute of Geology, Zagreb (1966): Federal Geological Institute, Beograd
Hunting ER, Kampfraath AA (2013) Contribution of bacteria to redox potential (Eh) measurements in sediments. Int J Environ Sci Technol 10:55–62
Hüttel M, Gust G (1992) Solute release mechanisms from confined sediment cores in stirred benthic chambers. Mar Ecol Prog Ser 82:187–197
Ivanović A, Sakač K, Marković B, Sokač B, Šušnjar M, Nikle L, Šušnjara A (1973a) Basic Geological Map SFRY 1:100 000, Sheet Obrovac L33–140, [in Croatian], Institute of Geology, Zagreb (1962-1967): Federal Geological Institute, Beograd
Ivanović A, Sakač K, Sokač B, Vrsalović-Carević I, Zupanić J (1973b) Basic Geological Map SFRY 1:100 000, Geology of sheet Obrovac [in Croatian], Institute of Geology, Zagreb (1967): Federal Geological Institute, Beograd
Juračić M, Crmarić R (2003) Holocene sediments and sedimentation in estuaries eastern Adriatic coast [in Croatian]. 3rd Croatian conference on water pp 227–233
Kay JT, Conklin MH, Fuller CC, O'Day PA (2001) Processes of nickel and cobalt uptake by a manganese oxide forming sediment in Pinal Creek, globe mining district, Arizona. Environ Sci Technol 35:4719–4725
Kelly SD, Newville MG, Cheng L, Kemner KM, Sutton SR, Fenter P, Sturchio NC, Spötl C (2003) Uranyl incorporation in natural calcite. Environ Sci Technol 37:1284–1287
Li Y-H, Gregory S (1974) Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38:703–714
Lohse L, Epping EHG, Helder W, van Raaphorst W (1996) Oxygen pore water profiles in continental shelf sediments of the North sea: turbulent versus molecular diffusion. Mar Ecol Prog Ser 145:63–75
Majcen Ž, Korolija B (1970) Basic Geological Map SFRJ 1:100 000, Geology of sheet Zadar. Institute of Geology, Zagreb (1967): Federal Geological Institute, Beograd (in Croatian)
Majcen Ž, Korolija B, Sokač B, Nikler L (1970) Basic Geological Map SFRY 1:100 000, Sheet Zadar L33–139 [in Croatian]. Institute of Geology, Zagreb (1963–1969): Federal Geological Institute, Beograd
Morford JL, Emerson SR, Breckel EJ, Kim SH (2005) Diagenesis of oxyanions (V, U, Re, and Mo) in pore waters and sediments from a continental margin. Geochim Cosmochim Acta 69(21):5021–5032
O’Connor AE, Luek JL, McIntosh H, Beck AJ (2015) Geochemistry of redox-sensitive trace elements in a shallow subterranean estuary. Mar Chem 172:70–81
Pfeifer K, Kasten S, Hensen C, Schulz HD (2001) Reconstruction of primary productivity from the barium contents in surface sediments of the South Atlantic Ocean. Mar Geol 177:13–24
Prifti E, Kaberi H, Zeri C, Rousselaki E, Michalopoulos P, Dassenakis M (2015) Calculation of benthic fluxes of metals using the pore water metal concentrations and the results from incubation experiments. 11th Panhellenic Symposium on Oceanography and Fisheries, Mytilene, Lesvos island, Greece, 341–344
Rasmussen H, Jorgensen BB (1992) Microelectrode studies of seasonal oxygen uptake in a coastal sediment: role of molecular diffusion. Mar Ecol Prog Ser 81:289–303
Roje-Bonacci T, Bonacci O (2013) The possible negative consequences of underground dam and reservoir construction and operation in coastal karst areas: an example of the hydro-electric power plant (HEPP) Ombla near Dubrovnik (Croatia). Nat Hazards Earth Syst Sci 13:2041–2052
Schlanger SO (1988) Strontium storage and release during deposition and diagenesis of marine carbonates related to sea-level variations. In: Lerman A, Meybeck M (eds) Physical and chemical weathering in geochemical cycles. NATO ASI series (series C: mathematical and physical sciences), 251. Springer, Dordrecht
Simpson SL, Rochford L, Birch GF (2002) Geochemical influences on metal partitioning in contaminated estuarine sediments. Mar Freshw Res 53:9–17
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley-Interscience, New York
Turner A, Millward GE, Le Roux SM (2004) Significance of oxides and particulate organic matter in controlling trace metal partitioning in a contaminated estuary. Mar Chem 88:179–192
Ullman WJ, Aller RC (1982) Diffusion coefficients in nearshore marine sediments. Limnol Oceanogr 27:552–556
Van Den Berg GA, Loch JPG, Van Der Heijdt LM, Zwolsman JJG (1999) Mobilisation of heavy metals in contaminated sediments in the river Meuse, The Netherlands. Water Air Soil Pollut 116:567–586
Vanderborght JP, Wollast R, Billen G (1977) Kinetic models of diagenesis in disturbed sediments part I mass transfer properties and silica diagenesis. Limnol Oceanogr 22:787–793
Viličić D, Terzić S, Ahel M, Burić Z, Jasprica N, Carić M, Caput Mihalić K, Olujić G (2008) Phytoplankton abundance and pigment biomarkers in the oligotrophic, eastern Adriatic estuary. Environ Monit Assess 142:199–218
Ye S, Laws EA, Gambrell R (2013) Trace element remobilization following the resuspension of sediments under controlled redox conditions: City Park Lake, Baton Rouge, LA. Appl Geochem 28:91–99
Young LB, Harvey HH (1992) The relative importance of manganese and iron oxides and organic matter in the sorption of trace metals by surficial lake sediments. Geochim Cosmochim Acta 56:1175–1186
Acknowledgments
Financial support from the Croatian Science Foundation through the project IP-11-2013-7555 TRACESS is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Lionel Denis
Electronic supplementary material
ESM 1
(DOCX 47 kb)
Rights and permissions
About this article
Cite this article
Fiket, Ž., Fiket, T., Ivanić, M. et al. Pore water geochemistry and diagenesis of estuary sediments—an example of the Zrmanja River estuary (Adriatic coast, Croatia). J Soils Sediments 19, 2048–2060 (2019). https://doi.org/10.1007/s11368-018-2179-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2179-9