Abstract
Purpose
The aim of this study was to evaluate the effectiveness of soil amendments and foliar fertilizer on Cd and Pb immobilization in contaminated soils.
Materials and methods
A field experiment was conducted in contaminated soils, wherein four amendments (sepiolite (SE), single superphosphate (SSP), triple super phosphate (TSP), calcium magnesium phosphate (CMP)) in combination with one foliar fertilizer (ZnSO4) were investigated to reduce Cd and Pb bioavailability in calcareous soils. Total Cd and Pb concentrations in wheat, soil, and amendments were determined using inductively coupled plasma mass spectrometry. Available concentrations of Cd and Pb in soils were extracted using diethylenetriamine pentaacetic acid.
Results and discussion
The results indicated that application of these amendments and foliar fertilizer significantly decreased Cd availability in soils and Cd accumulation in wheat (P < 0.05); however, the soil amendments plus Zn fertilizer did not significantly decrease Cd and Pb concentrations in wheat. Compared with the control, application of soil amendments effectively reduced the available Cd and Pb in soils by 25.69~54.13% and 9.86~42.14%, respectively. Accordingly, the reduction of Cd and Pb concentrations in wheat grain by the soil amendments was 20.68~41.38% and 23.68~55.26%, respectively.
Conclusions
Among all the treatments, the addition of SE + SSP and SE + CMP exhibited the most efficiency in reducing Cd and Pb availability, respectively, in the soil.
Similar content being viewed by others
1 Introduction
Increasing anthropogenic activities, such as mining, smelting, irrigation using waste water, application of sewage sludge, and atmospheric deposition, have caused severe heavy metal contamination around the world (Wong et al. 2002). Soil can act as a source and as a sink for heavy metals. Accumulation of heavy metals in soils and subsequently in the food chain is a potential threat to human health (Foucault et al. 2013). Moreover, heavy metal uptake by crops is one of the major pathways for food-chain contamination and human exposure.
During recent decades, different remediation techniques have been developed to reduce total or bioavailable concentrations of heavy metals in soils and thus minimize their accumulation in the food chain (Shahid et al. 2012; Clemente et al. 2012). However, several soil remediation techniques, such as ex situ excavation (Li et al. 2011), verification (Boisson et al. 1999), and electrokinetics (Fan et al. 2010), have not been widely utilized because of their relatively high costs or level of soil disruption. Accordingly, practical application of these remediation techniques to arable soils is seriously limited, especially in China where low or moderate levels of heavy metal contamination are widespread and there is an increasing demand for food safety. As an alternative technique, in situ metal immobilization using soil amendments has been promoted as a rapid, cost-effective, and low disruption technique (Keller et al. 2005; Lee et al. 2009). Many amendments, such as organic additives (Beesley et al. 2014), phosphate minerals (Brown et al. 2004), clay minerals (Sun et al. 2013; Liang et al. 2014), and industrial and agricultural by-products (Bose and Bhattacharyya 2008; Wang et al. 2014) have been developed for immobilization of heavy metals in soils. These amendments decrease the availability of heavy metals in contaminated soils based on changes in physicochemical properties of the soil, like soil pH and organic matter content (Adams et al. 2004; Sauve et al. 2003). Several studies have demonstrated that in situ heavy metal immobilization, using phosphate minerals (Cao et al. 2009; Mignardi et al. 2012), clay minerals (Sun et al. 2013; Liang et al. 2014), lime (Geeblen et al. 2003), or organic matter (Karer et al. 2015), is a cost-effective and environmentally sustainable remediation technology. In addition, application of Zn fertilizer has been reported to decrease the accumulation of Cd in crops or vegetables. Yang et al. (2011) reported that application of foliar Zn or seed-soaking Zn fertilizers decreased Cd concentrations in cucumbers by 12–36%. Similarly, Zn fertilizers could decrease Cd concentrations in the shoots of bread and durum wheat (Green et al. 2003; Koleli et al. 2004). Therefore, the addition of amendments or extra fertilizers is a promising and sustainable strategy for remediation of heavy metal-contaminated soils, which can not only effectively immobilize Cd and Pb in soils but also improve soil properties.
Although several soil amendments have been extensively examined, most previous studies were limited to pot experiments. The effectiveness of soil amendments might not be comprehensively evaluated from the results of pot experiments. Consequently, in this study, a field experiment was carried out to examine the implementation of soil amendments combined with foliar fertilizer in arable soils. The main objective was to evaluate the effectiveness of soil amendments and foliar fertilizer on immobilization of Cd and Pb in contaminated soils.
2 Materials and methods
2.1 Experiment site
The field experiment was carried out in arable soil of Jiyuan City (112°31′21.319″E, 35°08′24.918″N), northwestern Henan Province, China. It has a typical temperate and monsoonal climate with average parameters as follows: temperature 14.9 °C, annual rainfall 600.3 mm, and relative humidity 68%. The frostless duration is 233 days per year.
There are several smelters, such as Yuguang, Wanyang, and Jilin smelters in Jiyuan City. The soils were contaminated by Cd and Pb because of atmospheric depositions from these smelters.
2.2 Soil amendments
Four soil amendments and one foliar fertilizer were selected because they were inexpensive and locally available. Four soil amendments included sepiolite (SE), single superphosphate (SSP), triple super phosphate (TSP), and calcium magnesium phosphate (CMP). One foliar fertilizer of Zn was supplied as ZnSO4. The main elemental composition of the soil amendments are given in Table 1.
2.3 Experimental design
A randomized complete block split-plot design was employed in this study. Each plot consisted of an area of 50 m2 (25 m long by 2 m wide); the plots were separated from each other by 20 cm to avoid possible inter-plot contamination. The field experiment arranged in Yiyuan City was showed in Table 2. The treatments in the experiments included (1) control (CON), (2) 0.5% SE (W/W), (3) 0.5% SSP (W/W), (4) 0.5% TSP (W/W), (5) 0.5% CMP (W/W), (6) 0.5% SE + 0.5% SSP, (7) 0.5% SE + 0.5% TSP, (8) 0.5% SE + 0.5% CMP, (9) 0.5% SE + 300 mg/L Zn, (10) 0.5% SSP + 300 mg/L Zn, (11) 0.5% TSP + 300 mg/L Zn, (12) 0.5% CMP + 300 mg/L Zn, (13) 0.5% SE + 0.5% SSP + 300 mg/L Zn, (14) 0.5% SE + 0.5% TSP + 300 mg/L Zn, (15) 0.5% SE + 0.5% CMP + 300 mg/L Zn. The level of 0.5% of soil amendments, i.e., 15 t/ha of soil amendments, on the basis of the recommendation level (1.5 t/ha) of phosphate for wheat, was applied before planting. A total of 45 plots were prepared with three replicates for each treatment. Soil amendments were spread on the soil surface and then plowed into the soil 30 days prior to sowing. The foliar fertilizer of Zn was sprayed at the grain filling stage.
2.4 Wheat cultivation
A common wheat cultivar was selected as a representative crop in this experiment. Wheat seeds (1.5 kg per plot) were sown on 24 Oct 2015, and then harvested on 3 June 2016.
The field was managed according to conventional operating practices. Irrigation was applied three times during the experiment.
2.5 Plant sampling and chemical analysis
Five subsamples of plants were collected from each plot, and then separated into roots, stem, leaves, and grains. The plant samples were packed in clean plastic bags and then transported to the laboratory.
The plant samples were washed thoroughly with tap water, followed by several washes with deionized water, and then oven-dried at 50–60 °C to a constant weight. The dried samples were ground with a stainless-steel grinder chamber (MM400, Retsch, Germany). Plant samples (0.5 g) were weighed and then digested using concentrated nitric acid (HNO3)–perchloric acid (HClO4). Total Cd and Pb concentrations in plant samples were determined using an inductively coupled plasma mass spectrometer (ICP-MS) (ELAN DRC-e, Perkin Elmer SCIEX).
2.6 Soil sampling and chemical analysis
For the analysis of the physicochemical properties of the tested soils, five subsamples of soils (0–20 cm) were collected from each plot at the time of sowing. The soil pH was determined in 1:2.5 soil/water suspensions after 0.5 h with a combination pH electrode. The total N, K, and P of the soils were analyzed using standard methods of agricultural chemicals and soils (Lu 1999). The total N, K, and P in the soils were determined by kjeldahl nitrogen distillation, Mo-Sb colorimetric method, and atomic absorption spectrometry, respectively. Soil organic matter was determined by wet digestion with K2Cr2O7/H2SO4, and the conversion factor for organic carbon into organic matter was taken as 1.724. Cation exchange capacity (CEC) was determined using the 1 mol/L ammonium acetate method at pH 7.0. All the soil samples were air-dried and passed through a 1-mm sieve, followed by the nitric acid (HNO3) and hydrogen peroxide (H2O2) hot block digestion procedure using Method 3050a of the USEPA. The total concentrations of Cd and Pb were determined using ICP-MS.
In addition, the soils adhering to the roots of wheat were collected in the process of plant sampling to determine the available Cd and Pb in soils. Available Cd and Pb concentrations in the soils were extracted using diethylene triamine pentaacetic acid (DTPA), and then determined using ICP-MS.
2.7 Quality control
Blank, replicates, and certified reference material (soil: GBW07406; wheat: GBW10035) obtained from China Standard Materials Research Center, Beijing, PR China, were used for quality control. Satisfactory precision and accuracy were required to be within ±20% and between 85 and 120%, respectively.
2.8 Statistical analysis
All the data were analyzed with SPSS 17.0 and Origin 8.0 for Windows. Differences between treatment means were tested with one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test.
3 Results and discussion
3.1 Physicochemical properties of soils in the field experiment
The soil had a high pH value of 8.21 (alkaline soil) and a moderate cation exchange capacity of 9.08 cmol/kg (+). The soil in the experiment area belongs to Ustic Cambosoil according to Chinese soil taxonomy. The organic matter and CaCO3 content of the soils were 17.8 and 37.8 g/kg, respectively. Total N, P, and K concentrations were 1.46, 0.80, and 18.9 g/kg, respectively. The geometric mean values of Fe, Mn, Al, and Ca were 27,290 ± 1.03, 554 ± 1.05, 39,910 ± 1.05, and 24,644 ± 1.13 mg/kg, respectively.
The total and DTPA-extractable Cd and Pb concentrations are shown in Table 3. Total Cd and Pb concentrations ranged from 0.21 to 2.89 mg/kg and from 142.6 to 212.2 mg/kg, with average values of 2.00 and 157.7 mg/kg, respectively. Cd concentration in the soils was three times higher than the limit value of 0.6 mg/kg (soil pH >7.5) (GB15618-1995). Although the concentration of Pb in the soils was lower than the limit value of 350 mg/kg (soil pH >7.5) (GB15618–1995), Pb accumulation in wheat grains was slightly higher than the maximum permissible concentration (MPC) recommended by the National Standard (GB 2762-2012) (Xing et al. 2016). The concentrations of DTPA-extractable Cd and Pb ranged between 0.87 and 1.15 mg/kg and between 39.50 and 69.32 mg/kg, respectively, with the average concentration of 1.09 and 60.73 mg/kg, respectively.
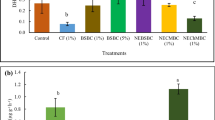
3.2 Changes in available heavy metals in the soil
The effect of soil amendments combined with foliar fertilizer on Cd and Pb availability in soils was shown in Fig. 1a, b, respectively. Compared with the control, application of soil amendments effectively reduced the available Cd and Pb in soils by 25.69~54.13% and 9.86~42.14%, respectively (P < 0.05). The lowest available Cd and Pb concentrations were found in the SE + SSP and SE + CMP treatments, respectively. The combination of sepiolite and SSP appeared significantly efficient than the single treatments in reducing availability of Cd and Pb in this study (P < 0.05), which was in agreement with the results reported by Wang et al. (2010). However, the reduction of availability Cd and Pb in the soils treated by the combination of sepiolite and other P materials were not significantly higher than the single treatment (P > 0.05). The results in this study demonstrated that addition of phosphates and clay materials caused immobilization of Cd and Pb in the rhizosphere soil.
Various mechanisms have been advanced to explain the immobilization of Cd and Pb by phosphate amendments. A dissolution–precipitation mechanism via the formation of pyromorphite-like mineral was used to explain the decrease in Pb availability in previous studies (Ma et al. 1995; Chen et al. 2007; Debela et al. 2013). Da Rocha et al. (2002) suggested that Cd immobilization could be associated with the ion exchange and complexation mechanisms. Surface adsorption or fixation mechanisms involving the formation of Cd-phosphate on the surface of the amendment were also reported in several studies (Marchat et al. 2007; Matusik et al. 2008; Corami et al. 2008). However, Ma et al. (1994) reported Cd-phosphate minerals were not detected via X-ray diffraction (XRD) but Cd-Ca-phosphate might have been formed in the experiment.
Clay minerals are reported to decrease metal mobility in contaminated soil by changing soil properties such as soil pH (van Hervijnen et al. 2007). In this study, sepiolite contained a significant percentage of calcium carbonate (CaCO3) and had a high pH of 10.1. pH-dependent exchange sites on organic matter and oxide clay minerals can be ionized with increasing soil pH, which can increase the adsorption of Cd to soil colloids or soil clay particles (Tapia et al. 2010). Liang et al. (2014) also reported that precipitation of Cd as carbonates or hydroxides and surface complexation were the main immobilization mechanisms for sepiolite.
3.3 Cd and Pb concentrations in different parts of the wheat plant
Cd concentrations in different parts of wheat after addition of different amendments and foliar fertilizer are shown in Fig. 2a, b. As seen in Fig. 2a,b, Cd in wheat plant parts decreased in the order of root Cd > leaf Cd > stem Cd > grain Cd; the concentrations of Cd in each plant part differed from each other significantly (P < 0.05), which indicated the slight ability of wheat to transport Cd within the plant. Compared with the control, Cd in the wheat roots treated with different amendments were significantly different (P < 0.05), except for those treated with CMP and CMP + Zn. Cd concentrations in the stems and leaves of wheat plants treated with different amendments were lower than those in the control. The addition of SE, SE + Zn, SSP, SSP + Zn, SE + SSP, and SE + TSP to the soils significantly decreased Cd concentrations in wheat grains compared with the control (Fig. 2b). The addition of SE, SSP, TSP, and CMP to the soils decreased the Cd concentration in wheat grains by 24.14, 34.48, 27.58, and 20.68%, respectively, when compared with the control. The combination amendment treatments SE + SSP, SE + TSP, and SE + CMP to the soils decreased the Cd concentration in wheat grains by 41.38, 34.48, and 31.72%, respectively, when compared with the control. Among all the treatments, the addition of SE + SSP to the soils effectively decreased the Cd accumulation in the wheat. Similar results were reported by Wang et al. (2008), who found that cabbage shoot Cd decreased by 16.5~66.9% after addition of phosphate amendments.
Pb concentrations in different parts of wheat under different treatments are shown in Fig. 3a, b. As seen in Fig. 3a, b, most of Pb was sequestered in wheat roots rather than the aboveground parts under the different treatments, which was in agreement with Shahid et al. (2014) who found that the presence of phosphate amendments increased Pb sequestration in roots of both pea and tomato. Compared with the control treatment, the addition of SE, SSP, TSP, and CMP to the soils significantly decreased the Pb concentration in the wheat grains by 34.21, 36.84, 28.94, and 50% (Fig. 3b) (P < 0.05), respectively. Among the soil amendments, the addition of SE + CMP to the soils mostly decreased the Pb accumulation in wheat grains by 55% (P < 0.05). Application of amendments significantly affected metal partitioning between roots and aboveground parts. Cao et al. (2002) reported that shoot tissue Pb concentrations in St. Augustine grass were reduced by 20 to 71% after application of phosphate materials to Pb-contaminated soil because of the formation of chloropyromorphite on the cell walls of roots, within the root rhizosphere and in the bulk soils. In addition, the formation of Pb–Ca-phosphates in the soils or on the root cell of cabbage might be responsible for decreasing Pb translocation from roots to shoots (Wang et al. 2008).
The addition of soil amendments combined with foliar fertilizer did not consistently decrease the Cd and Pb concentrations in wheat grains. Pb concentration in the wheat grains under the treatment with SE + Zn, SE + TSP + Zn, and SE + CMP + Zn were higher than those treated by SE, SE + TSP, and SE + CMP, respectively. Compared with the control, Cd concentrations in grains were reduced by 20.69, 31.04, 27.58, 24.14, 37.93, 24.14, and 31.03% for SE + Zn, SSP + Zn, TSP + Zn, CMP + Zn, SE + SSP + Zn, SE + TSP + Zn, and SE + CMP + Zn, respectively. Compared with the control, the addition of SE + Zn, SSP + Zn, TSP + Zn, CMP + Zn, SE + SSP + Zn, SE + TSP + Zn, and SE + CMP + Zn decreased the Pb concentrations in wheat grains by 31.58, 34.21, 31.58, 39.47%, 23.68, and 47.38%, respectively.
Previous studies have demonstrated that the uptake of Cd seems to occur mainly via certain transporters responsible for the uptake of essential elements like Zn2+ and Ca2+ (Clemens 2006; Verbruggen et al. 2009). Accordingly, Zn or Ca was used as an antagonist to decrease Cd uptake in the plant. In this study, the addition of foliar fertilizer (Zn) did not consistently decrease the Cd uptake of wheat, which was in accord with Feng et al. (2013) who suggested that the spray of foliar fertilizer to additive-treated pakchoi (Brassica rapa L. chinensis) showed positive effects only under given conditions. However, Li et al. (2014) reported that the amendments combined with Zn fertilization further decreased water extractable Cd concentration from 34 to 84% in vegetables, such as spinach, radish, cabbage, and tomato.
3.4 Wheat yield
Biomass can be used as a useful indicator for the overall health of plant growing on the Cd- and Pb-contaminated soil. During the whole growing season, no wheat plants showed obvious symptoms of Cd toxicity. In addition, the positive effect of nutrient availability and reduction in the bioavailability of Pb and Cd in the soils had an effect on the yield of wheat under different treatments compared to the control. Wheat grown in artificial fertilizer-treated soils, while wheat grown in SE- and SE + Zn-treated soils showed minimum increment in the yield (Fig. 4). The wheat in the soils added by SE + SSP had the largest increase of yield with 35.5% increase over the control, while the wheat yield in the soils applied by SE had the least increase at 4.28%.
3.5 Translocation factor and bioconcentration factor
The translocation factor (TF) and bioconcentration factor (BCF) of Cd and Pb in wheat under different amendments are shown in Table 4. TF was calculated by the ratio of the concentration of heavy metals in wheat grains and the concentration of heavy metals in root. BCF was defined as the ratio of the heavy metals in the edible plant parts to the concentrations of heavy metals in soils.
Translocation ratio of heavy metals varied among all the treatments that may be because the translocation of metals is a metabolic process controlled by the physicochemical condition of the soil. As seen in Table 4, application of all the amendments and foliar fertilizer decreased the TF of Pb in wheat, while the TF of Cd increased when treated with SE, SE + Zn, TSP + Zn, SE + TSP, and SE + TSP + Zn compared with the control. The TFs were less than 1 for Cd and Pb treated with different amendments, suggesting slight ability of wheat to transport heavy metals. The lowest TFs of Cd and Pb were found in wheat with the addition of SE + SSP and SE + CMP, respectively. The translocation ratio of Cd and Pb varied among all the treatments because of the metabolic processes controlled by the physicochemical properties of the soils. The translocation of heavy metals in wheat followed the order Cd > Pb, which indicated that heavy metals in soils were not evenly absorbed by plants and that translocation was not only related to the concentrations of heavy metals in soils. Similar results were reported by Castaldi et al. (2009) who found that translocation for wheat and pea followed the order Cd > Pb. High Cd translocation was found in plants because of its similar properties to Zn (Liphadzi and Krikham 2006), and Cd translocation in wheat (Triticum aestivum L.) was regulated by Zn (Green et al. 2003). Low translocation of Pb was found in plants because Pb was sequestered in the vacuole or cell walls in the root (Castaldi et al. 2009), which was related to stability property of Pb in soil-plant system. Sanita di Toppi and Gabbrielli (1999) also reported that Pb was early recognized by the plant roots as a toxic compound, and sequestrated in the vacuole or in the cell walls, thus leading to low translocation in the shoots.
BCF represents the transfer potential of heavy metals from the soil to the plants, which depends on the properties of the metals and soils (Singh et al. 2010). The trend in the BCF showed low transfer of Cd and Pb in wheat treated with different amendments, which indicated low availability of heavy metals in the amended soils compared with the control. BCF was the lowest in SE + SSP-treated soils for Cd, while the lowest BCF of Pb was found in SE + CMP-treated soils.
4 Conclusions
Results from this field experiment indicated that addition of sepiolite and phosphates to contaminated soil decreased the Cd and Pb availability in the soils, which in turn promoted plant growth, and thus decreased the Cd and Pb accumulation in wheat. Amendments of SE + SSP and SE + CMP were the most effective in reducing Cd and Pb availability, respectively, in soils. Compared with the control, application of soil amendments effectively reduced the available Cd and Pb in soils by 25.69~54.13% and 9.86~42.14%, respectively. The addition of SE + SSP mostly decreased the Cd concentration in wheat by 41.38%, while the addition of SE + CMP significantly decreased the Pb accumulation in the wheat grains by 55%. Unexpectedly, the addition of Zn fertilizer did not promote the reduction of Cd and Pb availability in soils and accumulation in wheat. The TFs were less than 1 for Cd and Pb treated with different amendments, which indicated low translocation of wheat. BCFs of wheat for Cd and Pb were lowest in SE + SSP- and SE + CMP-treated soils, respectively. The results from this study showed that the use of soil amendments may represent a key factor to remediation of soils.
References
Adams ML, Zhao FJ, McGrath SP, Nicholson FA, Chambers BJ (2004) Predicting cadmium concentrations in wheat and barley grain using soil properties. J Environ Qual 33:532–541
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson J (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202
Boisson J, Ruttens A, Mench M, Vangronsveld J (1999) Immobilization of trace metals and arsenic by different soil additives: evaluation by means of chemical extractions. Comm Soil Sci Plant Anal 30:365–387
Bose S, Bhattacharyya AK (2008) Heavy metal accumulation in wheat plant in soil amended with industrial sludge. Chemosphere 70:1264–1272
Brown S, Chaney R, Hallfrisch J, Ryan JA, Berti WR (2004) In situ soil treatments to reduce the phyto- and bioavailability of lead, zinc, and cadmium. J Environ Qual 33:522–531
Cao XD, Ma LQ, Chen M, Singh SP, Harris WG (2002) Impacts of phosphate amendments on lead biogeochemistry at a contaminated site. Environ Sci Technol 36(24):5296–5304
Cao XD, Wahbi A, Ma L, Li B, Yang YL (2009) Immobilization of Zn, Cu and Pb in contaminated soils using phosphate rock and phosphoric acid. J Hazard Mater 164:555–564
Castaldi P, Melis P, Silvetti M, Deiana P, Garau G (2009) Influence of pea and wheat growth on Pb, Cd, and Zn mobility and soil biological status in a polluted amended soil. Geoderma 151:241–248
Chen SB, Xu MG, Ma YB, Yang JC (2007) Evaluation of different phosphate amendments on availability of metals in contaminated soil. Ecotoxicol Environ Saf 67:278–285
Clemens S (2006) Toxic metal accumulation response to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Clemente R, Walker DJ, Pardo T, Martineze-Fernandez D, Bernal MP (2012) The use of halophytic plant species and organic amendments for the remediation of a trace elements-contaminated soil under semi-arid conditions. J Hazard Mater 223-224:63–71
Corami A, Mignardi S, Ferrini V (2008) Cadmium removal from single- and multi-metal (Cd plus Pb plus Zn plus Cu) solutions by sorption on hydroxyapatite. J Colloid Interf Sci 317:40–408
Da Rocha NCC, Decampos RC, Rossi AM, Moreira EL, Barbosa AF, Moure GT (2002) Cadmium uptake by hydroxyapatite synthesized in different conditions and submitted to thermal treatment. Environ Sci Technol 36:1630–1635
Debela F, Arocena JM, Thring RW, Whitcombe T (2013) Organic acids inhibit the formation of pyromorphite and Zn-phosphate in phosphorous amended Pb- and Zn-contaminated soil. J Environ Manag 116:156–162
Fan GP, Cang L, Xu H, Zhou DM, Zhou LX (2010) Enhanced electrokinetic remediation of heavy metals-organic pollutants compound contaminated red soils. J Agro-Environ Sci 29:1098–1104 (in Chinese)
Feng RW, Qiu WW, Lian F, Yu ZH, Yang YX, Song ZG (2013) Field evaluation of in situ remediation of Cd-contaminated soil using four additives, two foliar fertilizers and two varieties of pakchoi. J Environ Manag 124:17–24
Foucault Y, Durand MJ, Tack K, Schreck E, Geret F, Leveque T, Pradere P, Goxi S, Gumat C (2013) Use of ecotoxicity test and ecoscores to improve the management of polluted soils: case of secondary lead smelter plant. J Hazard Mater 246-247:291–299
Geeblen W, Adriano DC, Van Der Lelie D, Mench M, Carleer R, Clijsters H, Vangronsveld J (2003) Selected bioavailability assays to test the efficacy of amendment-induced immobilization of lead in soil. Plant Soil 249:217–228
Green CE, Chaney RL, Bouwkamp J (2003) Interactions between cadmium uptake and phytotoxic levels of zinc in hard red spring wheat. J Plant Nutr 26:417–430
Karer J, Wawra A, Zehetner F, Dunst G, Wagner M, Pavel PB, Puschenreiter M, Friesl-Hanl W, Soja G (2015) Effects of biochars and compost mixtures and inorganic additives on immobilisation of heavy metals in contaminated soils. Water Air Soil Pollut 226:342–354
Keller C, Marchetti M, Rossi L, Lugon-Moulin N (2005) Reduction of cadmium availability to tobacco (Nicotiana tabacum) plants using soil amendments in low cadmium-contaminated agricultural soils: a pot experiment. Plant Soil 276:69–84
Koleli N, Eker S, Cakmak I (2004) Effect of zinc fertilization on cadmium toxicity in durum and bread wheat grown in zinc-deficient soil. Environ Pollut 131:453–459
Lee SH, Lee JS, Choi YJ, Kim JG (2009) In situ stabilization of cadmium, lead, and zinc contaminated soil using various amendments. Chemosphere 77:1069–1075
Li DD, Hao XZ, Zhou DM, Zhan XH (2011) Remediation of chromium residue contaminated soil using a washing technology. J Agro-Environ Sci. 30(12):2451–2457
Li B, Yang JX, Wei DP, Chen SB, Li JM, Ma YB (2014) Field evidence of cadmium phytoavailability decreased effectively by rape straw and/or red mud with zinc sulphate in a Cd-contaminated calcareous soil. PLoS One 10:1–7
Liang XF, Han J, Xu YM, Sun YB, Wang L, Tan X (2014) In situ field-scale remediation of Cd polluted paddy soil using sepiolite and palygorskite. Geoderma 235-236:9–18
Liphadzi MS, Krikham MB (2006) Availability and plant uptake of heavy metals in EDTA-assisted phytoremediation of soil and composted biosolids. S Afr J Bot 72:391–397
Lu RK (1999) Analytical methods of agricultural chemistry in soil. China Agricultural Scinetech, Beijing
Ma QY, Logan TJ, Traina SJ (1994) Effects of aqueous Al, Cd, Cu, Fe(II), Ni, and Zn on Pb immobilization by hydroxyapatite. Environ Sci Technol 28:1219–1228
Ma QY, Logan TJ, Traina SJ (1995) Lead immobilization from aqueous solutions and contaminated soils using phosphate rocks. Environ Sci Technol 29:1118–1126
Marchat D, Bernache-Assollant D, Champion E (2007) Cadmium fixation by synthetic hydroxyapatite in aqueous solution-thermal behavior. J Hazard Mater 139:453–460
Matusik J, Bajda T, Manecki M (2008) Immobilization of aqueous cadmium by addition of phosphates. J Hazard Mater 152:1332–1339
Mignardi S, Corami A, Ferrini V (2012) Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 86:354–360
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Sauve S, Manna S, Turmel MC, Roy AG, Courchesne F (2003) Solid-solution partitioning of Cd, Cu, Ni, Pb, and Zn in the organic horizons of a forest soil. Environ Sci Technol 37:5191–5196
Shahid M, Arshad M, Kaemmerer M, Pinelli E, Probst A, Baque D, Pradere P, Dumat C (2012) Long term field metal extraction by pelargonium: phytoextraction efficiency in relation to plant maturity. Int J Phytoremediat 14:493–505
Shahid M, Xiong TT, Msood N, Leveque T, Quenea K, Austruy A, Foucault Y, Dumat C (2014) Influence of plant species and phosphorus amendments on metal speciation and bioavailability in a smelter impacted soil: a case study of food-chain contamination. J Soils Sediments 14:655–665
Singh A, Agrawal M, Marshall FM (2010) The role of organic vs. inorganic fertilizers in reducing phytoavailability of heavy metals in a wastewater-irrigated area. Ecol Eng 36:1733–1740
Sun YB, Sun GH, Xu YM, Wang L, Liang XF, Lin DS (2013) Assessment of sepiolite for immobilization of cadmium-contaminated soils. Geoderma 193-194:149–155
Tapia Y, Cala V, Eymar E, Frutos I, Garate A, Masaguer A (2010) Chemical characterization and evaluation of composts as organic amendments for immobilizing cadmium. Bioresource Technol 101:5437–5443
Van Hervijnen R, Hutchings TR, Al Tabbaa A, Moffat AJ, Johns ML, Ouki SK (2007) Remediation of metal contaminated soil with mineral-amended composts. Environ Pollut 150:347–354
Verbruggen M, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Wang BL, Xie ZM, Chen JJ, Jiang JT, Su QF (2008) Effects of field application of phosphate fertilizers on the availability and uptake of lead, zinc and cadmium by cabbage (Brassica chinenisis L.) in a mining tailing contaminated soil. J Environ Sci 202:1109–1117
Wang L, Xu YM, Sun Y, Liang XF, Qin X (2010) Immobilization of cadmium contaminated soils using natural clay mineral. J Safe Environ 10:35–38
Wang FL, Ouyang W, Hao FF, Lin CY, Song NN (2014) In situ remediation of cadmium-polluted soil reusing four by-products individually and in combination. J Soils Sediments 14:451–461
Wong SC, Li XD, Zhang G, Qi SH, Min YS (2002) Heavy metals in agricultural soils of the Peral River Delta, South China. Environ Pollut 119:33–44
Xing WH, Zhang HY, Scheckel KG, Li LP (2016) Heavy metal and metalloid concentrations in components of 25 wheat (Triticum aestivum) varieties in the vicinity of lead smelters in Henan Province, China. Environ Monit Assess 188:23–33
Yang JX, Wang LQ, Wei DP, Chen SB (2011) Foliar spraying and seed soaking of zinc fertilization decreased cadmium accumulation in cucumbers grown in Cd-contaminated soils. Soil Sediment Contam 20:400–410
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation (Grant Nos. 2015M580130, 2016T90126).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Winfried Schroeder
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Guo, G., Lei, M., Chen, T. et al. Evaluation of different amendments and foliar fertilizer for immobilization of heavy metals in contaminated soils. J Soils Sediments 18, 239–247 (2018). https://doi.org/10.1007/s11368-017-1752-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1752-y