Abstract
Purpose
Stripping contaminants from sediments with granular activated carbon (GAC) is a promising remediation technique in which the effectiveness depends on the rate of contaminant extraction from the sediment by the GAC. The purpose of the present study was to investigate the effect of mixing intensity on the short-term extraction rate of polycyclic aromatic hydrocarbons (PAHs) from contaminated sediment.
Materials and methods
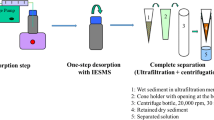
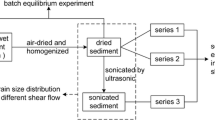
PAH desorption from sediment at a wide range of rotational speeds (min−1; rotations per minute (rpm)) was monitored by uptake in Tenax polymeric resins using a completely mixed batch reactor. Desorption data were interpreted using a radial diffusion model. Desorption parameters obtained with the radial diffusion model were correlated with particle size measurements and interpreted mechanistically.
Results and discussion
Fast desorption rate constants, D e /r 2, with D e the effective diffusion coefficient and r the particle radius, ranged from 3.7 × 10−3 to 1.1 × 10−1 day−1 (PHE) and 6 × 10−6 to 1.9 × 10−4 day−1 (CHR), respectively, and increased with the intensity of mixing. The D e /r 2 values would correspond to D e ranges of 1.8 × 10−14–1.2 × 10−16 m2 × day−1 and 1.8 × 10−12–3.7 × 10−15 m2 × day−1, assuming fast desorption from the measured smallest particle size (9 μm) classes at 200 and 600 rpm, respectively.
Conclusions
Desorption of PAHs was significantly accelerated by a reduction of particle aggregate size caused by shear forces that were induced by mixing. The effective intra-particle diffusion coefficients, D e , were larger at higher mixing rates.




Similar content being viewed by others
References
Ahn S, Werner D, Karapanagioti HK, McGlothlin DR, Zare RN, Luthy RG (2005) Phenanthrene and pyrene sorption and intraparticle diffusion in polyoxymethylene, coke, and activated carbon. Environ Sci Technol 39:6516–6526
Bird RB, Stewart WE, Lightfoot E (1960) Transport phenomena, 1st edition. Wiley, New York
Chai YZ, Kochetkov A, Reible DD (2006) Modeling biphasic sorption and desorption of hydrophobic organic contaminants in sediments. Environ Toxicol Chem 25:3133–3140
Cornelissen G, van Noort PCM, Govers HAJ (1997) Desorption kinetics of chlorobenzenes, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls: sediment extraction with Tenax (R) and effects of contact time and solute hydrophobicity. Environ Toxicol Chem 16:1351–1357
Cornelissen G, Rigterink H, van Noort PCM, Govers HAJ (2000) Slowly and very slowly desorbing organic compounds in sediments exhibit Langmuir-type sorption. Environ Toxicol Chem 19:1532–1539
Cornelissen G, Rigterink H, ten Hulscher DEM, Vrind BA, van Noort PCM (2001) A simple Tenax® extraction method to determine the availability of sediment-sorbed organic compounds. Environ Toxicol Chem 20:706–711
Cornelissen G, Gustafsson O, Bucheli TD, Jonker MTO, Koelmans AA, Van Noort PCM (2005) Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol 39:6881–6895
De Maagd PG-J, ten Hulscher DTEM, van den Heuvel H, Opperhuizen A, Sijm DTHM (1998) Physicochemical properties of polycyclic aromatic hydrocarbons: aqueous solubilities, n-octanol/water partition coefficients, and Henry’s law constants. Environ Toxicol Chem 17:251–257
Ghosh U, Talley JW, Luthy RG (2001) Particle-scale investigation of PAH desorption kinetics and thermodynamics from sediment. Environ Sci Technol 35:3468–3475
Ghosh U, Luthy RG, Cornelissen G, Werner D, Menzie CA (2011) In-situ sorbent amendments: a new direction in contaminated sediment management. Environ Sci Technol 45:1163–1168
Gustafsson O, Gschwend PM (1997) Soot as a strong partition medium for polycyclic aromatic hydrocarbons in aquatic systems. Molecular Markers in Environmental Geochemistry 671:365–381
Gustafsson O, Haghseta F, Chan C, MacFarlane J, Gschwend PM (1997) Quantification of the dilute sedimentary soot phase: implications for PAH speciation and bioavailability. Environ Sci Technol 31:203–209
Hale SE, Werner D (2010) Modeling the mass transfer of hydrophobic organic pollutants in briefly and continuously mixed sediment after amendment with activated carbon. Environ Sci Technol 44:3381–3387
Janssen EML, Beckingham BA (2013) Biological responses to activated carbon amendments in sediment remediation. Environ Sci Technol 47:7595–7607
Johnson MD, Keinath TM, Weber WJ (2001) A distributed reactivity model for sorption by soils and sediments. 14. Characterization and modeling of Phenanthrene desorption rates. Environ Sci Technol 35:1688–1695
Jonker MTO, Koelmans AA (2001) Polyoxymethylene solid phase extraction as a partitioning method for hydrophobic organic chemicals in sediment and soot. Environ Sci Technol 35:3742–3748
Jonker MTO, Smedes F (2000) Preferential sorption of planar contaminants in sediments from Lake Ketelmeer, the Netherlands. Environ Sci Technol 34:1620–1626
Koelmans AA, Lijklema L (1992) Sorption of 1,2,3,4-tetrachlorobenzene to sediments: the application of a simple three phase model. Chemosphere 25:313–325
Koelmans AA, Prevo L (2003) Production of dissolved organic carbon in aquatic sediment suspensions. Water Res 37:2217–2222
Koelmans AA, Jonker MTO, Cornelissen G, Bucheli TD, Van Noort PCM, Gustafsson O (2006) Black carbon: the reverse of its dark side. Chemosphere 63:365–377
Koelmans AA, Poot A, De Lange HJ, Velzeboer I, Harmsen J, van Noort PCM (2010) Estimation of in situ sediment-to-water fluxes of polycyclic aromatic hydrocarbons, polychlorobiphenyls and polybrominated diphenylethers. Environ Sci Technol 44:3014–3020
Kukkonen JVK, Landrum PF, Mitra S, Gossiaux DC, Gunnarsson J, Weston D (2003) Sediment characteristics affecting desorption kinetics of select PAH and PCB congeners for seven laboratory spiked sediments. Environ Sci Technol 37:4656–4663
Kupryianchyk D, Rakowska MI, Roessink I, Reichman EP, Grotenhuis JTC, Koelmans AA (2013) In situ treatment with activated carbon reduces bioaccumulation in aquatic food chains. Environ Sci Technol 47:4563–4571
Kupryianchyk D et al. (2015) Positioning activated carbon amendment technologies in a novel framework for sediment management. Integr Enviro Assess Manage 11:221–234
Maruya KA, Risebrough RW, Horne AJ (1996) Partitioning of polynuclear aromatic hydrocarbons between sediments from San Francisco Bay and their porewaters. Environ Sci Technol 30:2942–2947
McGroddy SE, Farrington JW (1995) Sediment Porewater partitioning of polycyclic aromatic hydrocarbons in three cores from Boston Harbor, Massachusetts. Environ Sci Technol 29:1542–1550
McLeod PB, Van den Heuvel-Greve MJ, Luoma SN, Luthy RG (2007) Biological uptake of polychlorinated biphenyls by Macoma balthica from sediment amended with activated carbon. Environ Toxicol Chem 26:980–987
Mulder H, Breure AM, Rulkens WH (2001) Application of a mechanistic desorption-biodegradation model to describe the behavior of polycyclic aromatic hydrocarbons in peat soil aggregates. Chemosphere 42:285–299
NEN 5733 (1997) Soil-determination of mineral oil content in soil and sediments with gas chromatography”
Noordkamp ER, Grotenhuis JTC, Rulkens WH (1997) Selection of an efficient extraction method for the determination of polycyclic aromatic hydrocarbons in contaminated soil and sediment. Chemosphere 35:1907–1917
Patmont CR et al. (2015) In situ sediment treatment using activated carbon: a demonstrated sediment cleanup technology. Integr Enviro Assess Manage 11:195–207
Pignatello JJ, Xing BS (1996) Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol 30:1–11
Poot A, Jonker M, Gillissen F, Koelmans AA (2014) Explaining PAH desorption from sediments using rock Eval analysis. Environ Pollut 193:247–253
Rakowska MI, Kupryianchyk D, Harmsen J, Grotenhuis T, Koelmans AA (2012) In situ remediation of contaminated sediments using carbonaceous materials. Environ Toxicol Chem 31:693–704
Rakowska M, Kupryianchyk D, Grotenhuis T, Rijnaarts H, Koelmans A (2013) Extraction of sediment-associated polycyclic aromatic hydrocarbons with granular activated carbon. Environ Toxicol Chem 32:304–311
Rakowska M, Kupryianchyk D, Smit M, Grotenhuis T, Rijnaarts H, Koelmans A (2014a) Kinetics of hydrophobic organic contaminant extraction from sediment by granular activated carbon. Water Res 51:86–95
Rakowska MI, Kupryianchyk D, Smit MPJ, Koelmans AA, Grotenhuis JTC, Rijnaarts HHM (2014b) Corrigendum to “kinetics of hydrophobic organic contaminant extraction from sediment by granular activated carbon” [Water Res 51 86–95]. Water Res 57:339
Rockne KJ, Shor LM, Young LY, Taghon GL, Kosson DS (2002) Distributed sequestration and release of PAHs in weathered sediment: the role of sediment structure and organic carbon properties. Environ Sci Technol 36:2636–2644
Schwarzenbach RP, Gschwend PM, Imboden DM (2002) Environmental organic chemistry. Wiley-Interscience
Smit MJ, Grotenhuis T, Bruning H, Rulkens W (2008) Desorption of Dieldrin from field aged sediments: simulating flood events. J Soils Sediments 8:80–85
Smit MPJ, Grotenhuis T, Bruning H, Rulkens WH (2010) Modeling desorption kinetics of a persistent organic pollutant from field aged sediment using a bi-disperse particle size distribution. J Soils Sediments 10:119–126
Spicer PT, Pratsinis SE (1996) Coagulation and fragmentation: universal steady-state particle-size distribution. AICHE J 42:1612–1620
Ten Hulscher TE, Vrind B, Van den Heuvel H, Van der Velde L, Van Noort P, Beurskens J, Govers H (1999) Triphasic desorption of highly resistant chlorobenzenes, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons in field contaminated sediment. Environ Sci Technol 33:126–132
van Noort PC, Poot A, Koelmans AA (2014) Analysis of organic contaminant desorption kinetic data for sediments and soils: implications for the Tenax extraction time for the determination of bioavailable concentrations. Sci Total Environ 490:235–238
Weber WJ, Kim HS (2005) Optimizing contaminant desorption and bioavailability in dense slurry systems. 1. Rheology, mechanical mixing, and PAH desorption. Environ Sci Technol 39:2267–2273
Weert J, Streminska M, Hua D, Grotenhuis T, Langenhoff A, Rijnaarts H (2010) Nonylphenol mass transfer from field-aged sediments and subsequent biodegradation in reactors mimicking different river conditions. J Soils Sediments 10:77–88
Werner D, Ghosh U, Luthy RG (2006) Modeling polychlorinated biphenyl mass transfer after amendment of contaminated sediment with activated carbon. Environ Sci Technol 40:4211–4218
Wu SC, Gschwend PM (1986) Sorption kinetics of hydrophobic organic compounds to natural sediments and soils. Environ Sci Technol 20:717–725
Acknowledgments
The present study was funded by the Dutch Technology Foundation STW, project no. 10030. We thank Erik Reichman for practical assistance during extraction procedures. We acknowledge financial support from Alterra, RIVM, Boskalis Dolman, and Norit. Deltares and De Vries and Van de Wiel are acknowledged for their contributions to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Karl J. Rockne
Electronic supplementary material
ESM 1
(PDF 717 kb)
Rights and permissions
About this article
Cite this article
Rakowska, M.I., Smit, M.P.J., Kupryianchyk, D. et al. Turbulent mixing accelerates PAH desorption due to fragmentation of sediment particle aggregates. J Soils Sediments 17, 277–285 (2017). https://doi.org/10.1007/s11368-016-1556-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1556-5