Abstract
Purpose
Studying the rate of chelant degradation is important to select environmental friendly compounds to assist phytoextraction. The objective of the present study was to evaluate degradation rate of complexes formed between synthetic or organic chelants and Pb aiming to increase the efficiency of phytoextraction while reducing adverse effects resulting from the Pb leaching.
Materials and methods
The following six chelating agents were tested: citric acid P.A., commercial citric acid, glutamic acid P.A., monosodium glutamate, nitrilotriacetic acid (NTA), and ethylenediaminetetraacetic acid (EDTA), besides a control treatment (no addition of chelating agent); they were applied at a concentration of 10 mmol dm−3 in pots containing 1 dm3 of Pb-contaminated soil.
Results and discussion
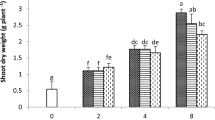
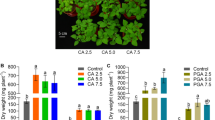
The results of this study showed that commercial citric acid adequately solubilized Pb to levels suitable for plant uptake and showed relatively rapid biodegradation in soil. Therefore, this commercial product may be a highly promising alternative for phytoextraction studies in the field. EDTA and NTA demonstrated high Pb solubilization ability but degraded comparatively slowly; therefore, they are not recommended for use in phytoextraction due to environmental risks regarding metal leaching.
Conclusions
The results of this study showed that commercial citric acid adequately solubilized Pb to levels suitable for plant uptake and showed relatively rapid biodegradation in soil, which is associated with a low risk of groundwater contamination. Therefore, this environmental friendly and low-cost product may be a highly promising alternative for inducing Pb phytoextraction.




Similar content being viewed by others
References
Andrade FV, Mendonça ES, Alvarez VVH, Novais RF (2003) Addition of organic and humic acids to latosols and phosphate adsorption effects. R Bras Ci Solo 27:1003–1011 (in Portuguese)
Arwidssona Z, Elgh-Dalgrenb K, Kronhelma T, Sjöbergc R, Allardb B, Hees P (2010) Remediation of heavy metal contaminated soil washing residues with amino polycarboxylic acids. J Hazard Mater 173:697–704
Babaeian E, Homaee M, Rahnemaie R (2015) Chelate-enhanced phytoextraction and phytostabilization of lead-contaminated soils by carrot (Daucus carota). Arch Agron Soil Sci 62:339–358
Bataillard P, Cambier P, Picot C (2003) Short term transformations of lead and cadmium compounds in soil after contamination. Eur J Soil Sci 54:1365–2389
Bucheli-Witschel M, Egli T (2001) Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol Rev 25:69–106
Chang J, Zhong Z, Xu H, Yao Z, Chen R (2013) Fabrication of poly (γ-glutamic acid)-coated Fe3O4 magnetic nanoparticles and their application in heavy metal removal. Chin J Chem Eng 21:1244–1250
EMBRAPA–Empresa Brasileira de Pesquisa Agropecuária (2011) Manual de métodos de análises de solo, 2nd edn. Embrapa Comunicação para Transferência de Tecnologia, Brasília
Evangelou MWH, Ebel M, Schaeffer A (2007) Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 68:989–1003
Evangelou MWH, Ebel M, Schaeffer A (2006) Evaluation of the effect of small organic acids on phytoextraction of Cu and Pb from soil with tobacco Nicotiana tabacum. Chemosphere 63:996–1004
Freitas EVS, Nascimento CWA, Souza AP, Silva FB (2013) Citric acid-assisted phytoextraction of lead: a field experiment. Chemosphere 92:213–217
Freitas EVS, Nascimento CWA (2009) The use of NTA for lead phytoextraction from soil from a battery recycling site. J Hazard Mater 171:833–837
Freitas EVS, Nascimento CWA, Biondi CM, Silva JPS, Souza AP (2009a) Lead desorption and leaching in a spodosol amended with chelant agents. R Bras Ci Solo 33:517–525 (in Portuguese)
Freitas EVS, Nascimento CWA, Silva AJ, Duda GP (2009b) Citric acid enhances lead phytoextraction from a soil contaminated by automotive batteries. R Bras Ci Solo 33:467–473 (in Portuguese)
Friesl W, Friedl J, Platzer K, Horak O, Gerzabek MH (2006) Remediation of contaminated agricultural soils near a former Pb/Zn smelter in Austria: batch, pot and field experiments. Environ Pollut 144:40–50
González I, Neaman A, Cortés A, Rubio P (2014) Effect of compost and biodegradable chelate addition on phytoextraction of copper by Oenothera picensis grown in Cu-contaminated acid soils. Chemosphere 95:111–115
Kari FG, Giger W (1995) Modeling the photochemical degradation of ethylenediaminetetraacetate in the river Glatt. Environ Sci Technol 29:2814–2827
Lan J, Zhang S, Lin H, Li T, Xu X, Li Y, Jia Y, Gong G (2013) Efficiency of biodegradable EDDS, NTA and APAM on enhancing the phytoextraction of cadmium by Siegesbeckia orientalis L. grown in Cd-contaminated soils. Chemosphere 91:1362–1367
Lewis AE, Beautement C (2002) Prioritising objectives for waste reprocessing: a case study in secondary lead refining. Waste Manag 22:677–685
Liu D, Islam E, Li T, Yang X, Jin X, Mahmood Q (2008) Comparison of synthetic chelating agents and low molecular weight organic acids in enhancing phytoextraction of heavy metals by two ecotypes of Sedum alfredii Hance. J Hazard Mater 153:114–122
Luo C, Shen Z, Li X (2005) Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59:1–11
Martell WE, Smith WM (1974) Critical stability constants: amino acids. Plenum Press, New York
Meers E, Tack FMG, Verloo MG (2008) Degradability of ethylenediaminedisuccinic acid (EDDS) in metal contaminated soils: implications for its use soil remediation. Chemosphere 70:358–363
Meers E, Lesage E, Lamsal S, Hopgood M, Vervaeke P, Tack FMG, Verloo MG (2005a) Enhanced phytoextraction: I. effect of EDTA and citric acid on heavy metal mobility in a calcareous soil. Int J Phytorem 7:129–142
Meers E, Ruttens A, Hopgood MJ, Samson D, Tack FMG (2005b) Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58:1011–1022
Meers E, Hopgood M, Lesage E, Vervaeke P, Tack FMG, Verloo MG (2004) Enhanced phytoextraction: search of EDTA alternatives. Int J Phytorem 6:95–109
Mehmood F, Rashid A, Mahmood T, Dawson L (2013) Effect of DTPA on Cd solubility in soil—accumulation and subsequent toxicity to lettuce. Chemosphere 90:1805–1810
Mera MF, Rubio M, Pérez CA, Galván V, Germanier A (2015) SR μXRF and XRD study of the spatial distribution and mineralogical composition of Pb and Sb species in weathering crust of corroded bullets of hunting fields. Microchem J 119:114–122
Nörtemann B (2005) Biodegradation of chelating agents: EDTA, DTPA, PDTA, NTA and EDDS. In: Nowack B, Vanbriesen JM (eds) Biogeochemistry of chelating agents. American Chemical Society, Washington, pp 150–170
Novozamsky I, Lexmond TM, Houba VJGA (1993) Single extraction procedure of soil for evaluation of uptake of some heavy metals by plants. Int J Environ Anal Chem 51:47–58
Parra R, Ulery A, Elless MP, Blaylock MJ (2008) Transient phytoextraction agents: establishing criteria for the use of chelants in phytoextraction of recalcitrant metals. Int J Phytorem 10:415–429
Pérez AM, Madrid F, Cabrera F, Madejón E (2007) Amendments and plant cover influence on trace element pools in a contaminated soil. Geoderma 139:1–10
Pires AMM, Mattiazzo ME (2007) Kinetics of heavy metal solubilization by organic acids in sludge-treated soils. R Bras Ci Solo 31:143–151 (in Portuguese)
Shimp RJ, Lapsins EV, Ventullo RM (1994) Chemical fate and transport in a domestic septic system: biodegradation of linear alkylbenzene sulfonate (LAS) and nitrilotriacetic acid (NTA). Environ Toxicol Chem 13:205–212
USEPA–United States Environmental Protection Agency (1998) Method 3051A—microwave assisted acid digestion of sediments, sludges, soils and oils. http://www.epa.gov/SW-846/pdfs/3051a.pdf
Van Devivere PC, Saveyn H, Verstraete W, Feijtel TCJ, Schowanek DR (2001) Biodegradation of metal–[S, S]-EDDS complexes. Environ Sci Technol 35:1765–1770
Zhao S, Lian F, Duo L (2011) EDTA-assisted phytoextraction of heavy metals by turfgrass from municipal solid waste compost using permeable barriers and associated potential leaching risk. Biores Technol 102:621–626
Acknowledgments
Senior author is grateful to CNPq for the scholarship during his graduate course (doctorate). Clístenes Nascimento is also grateful to CNPq for a research productivity scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jaume Bech
Rights and permissions
About this article
Cite this article
Freitas, E.V., Nascimento, C. Degradability of natural and synthetic chelating agents applied to a lead-contaminated soil. J Soils Sediments 17, 1272–1278 (2017). https://doi.org/10.1007/s11368-015-1350-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1350-9