Abstract
Purpose
This paper aims to investigate the effect of clay mineralogy on aggregate stability under the consideration of soil particle surface electric field in electrolyte solution.
Materials and methods
Aggregates of montmorillonite, kaolinite, and their compounds in different mass ratio were used as experimental materials. The degree to which the aggregates broke down under different electrolyte concentrations was evaluated both qualitatively and quantitatively. The aggregate breakdown process was observed with a video microscope. Furthermore, the percentage of particles (micro-aggregates and single particles) in diameter of <10, <5, and <2 μm to the total sample mass were measured based on Stokes equation.
Results and discussion
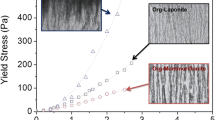
Different electrolyte concentrations correspond to different surface potential values around the particle surface. (1) The amount of released small particles for aggregate of clay compounds was several times higher than that for montmorillonite aggregate after breakdown. (2) Under high surface potential conditions, aggregate breakdown in a way of explosion was observed, and the critical surface potential values for the explosion increased with the increase of kaolinite content in aggregates. (3) The content of released particles for clay compound aggregates with high kaolinite content was much higher than that for aggregates with low kaolinite content under high surface potential values.
Conclusions
The coupling effects of electric field and clay mineralogy determined the aggregate stability of montmorillonite and clay compounds. This indicated that soil aggregates with relatively high silt content would be less stable than those with relatively high clay content under heavy rainfall; the most erosive soil might not be clay soils, but the silt soils, especially those under sudden storm after long-time drought.







Similar content being viewed by others
References
Agassi M, Morin J, Shainberg I (1985) Effect of raindrop impact energy and water salinity on infiltration rates of sodic soils. Soil Sci Soc Am J 49:186–190
Borda T, Celi L, Zavattaro L, Sacco D, Barberis E (2011) Effect of agronomic management on risk of suspended solids and phosphorus losses from soil to waters. J Soils Sediments 11:440–451
Derjaguin B, Landau L (1993) Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog Surf Sci 43:30–59
Ghadiri H, Hussein J, Rose CW (2007) A study of the interactions between salinity, soil erosion, and pollutant transport on three Queensland soils. Soil Res 45:404–413
Grasso D, Subramaniam K, Butkus M, Strevett K, Bergendahl J (2002) A review of non-DLVO interactions in environmental colloidal systems. Rev Environ Sci Biol 1:17–38
He Z, Wilson M, Campbell C, Edwards A, Chapman S (1995) Distribution of phosphorus in soil aggregate fractions and its significance with regard to phosphorus transport in agricultural runoff. Water Air Soil Pollut 83:69–84
Hou J, Li H, Zhu H, Wu L (2009) Determination of clay surface potential: a more reliable approach. Soil Sci Soc Am J 73:1658–1663
Hu F, Xu C, Li H, Li S, Yu Z, Li Y, He Y (2015) Particle interaction forces and their effects on soil aggregates breakdown. Soil Tillage Res 147:1–9
Jomaa S, Barry DA, Brovelli A, Sander GC, Parlange J-Y, Heng B, Tromp-van Meerveld H (2010) Effect of raindrop splash and transversal width on soil erosion: laboratory flume experiments and analysis with the Hairsine–Rose model. J Hydrol 395:117–132
Kuhn NJ, Armstrong EK (2012) Erosion of organic matter from sandy soils: solving the mass balance. Catena 98:87–95
Kuhn NJ, Armstrong EK, Ling AC, Connolly KL, Heckrath G (2012) Interrill erosion of carbon and phosphorus from conventionally and organically farmed Devon silt soils. Catena 91:94–103
Lado M, Ben-Hur M (2004) Soil mineralogy effects on seal formation, runoff and soil loss. Appl Clay Sci 24:209–224
Lal R, Shukla A (2004) Principles of soil physics. Marcel Dekker, Inc, New York, pp 48–55
Le Bissonnais Y (1996) Aggregate stability and assessment of soil crustability and erodibility: I. Theory and methodology. Eur J Soil Sci 47:425–437
Leng Y (2012) Hydration force between mica surfaces in aqueous KCl electrolyte solution. Langmuir 28:5339–5349
Li H, Peng X, Wu L, Jia M, Zhu H (2009) Surface potential dependence of the Hamaker constant. J Phys Chem C 113:4419–4425
Li H, Qing CL, Wei SQ, Jiang XJ (2004) An approach to the method for determination of surface potential on solid/liquid interface: theory. J Colloid Interface Sci 275:172–176
Li S, Li H, Xu CY, Huang XR, Xie DT, Ni JP (2013) Particle interaction forces induce soil particle transport during rainfall. Soil Sci Soc Am J 77:1563–1571
Liang Y, Hilal N, Langston P, Starov V (2007) Interaction forces between colloidal particles in liquid: theory and experiment. Adv Colloid Interf Sci 134–35:151–166
Liu XM, Li H, Du W, Tian R, Li R, Jiang XJ (2013) Hofmeister effects on cation exchange equilibrium: quantification of ion exchange selectivity. J Phys Chem C 117:6245–6251
Liu XM, Li H, Li R, Tian R, Hou J (2012) A new model for cation exchange equilibrium considering the electrostatic field of charged particles. J Soil Sediment 12:1019–1029
Marchuk A, Rengasamy P (2011) Clay behaviour in suspension is related to the ionicity of clay–cation bonds. Appl Clay Sci 53:754–759
Pansu M, Gautheyrou J (2006) Handbook of soil analysis: mineralogical, organic and inorganic methods. Springer, Berlin, pp 18–25
Sposito G (1984) The surface chemistry of soils. Oxford University Press, New York, pp 155–159
Suzuki S, Prayongphan S, Ichikawa Y, Chae B-G (2005) In situ observations of the swelling of bentonite aggregates in NaCl solution. Appl Clay Sci 29:89–98
Tian R, Yang G, Li H, Gao X, Liu X, Zhu H, Tang Y (2014) Activation energies of colloidal particle aggregation: towards a quantitative characterization of specific ion effects. Phys Chem Chem Phys 16:8828–8836
Van Dijk A, Meesters A, Bruijnzeel L (2002) Exponential distribution theory and the interpretation of splash detachment and transport experiments. Soil Sci Soc Am J 66:1466–1474
Verwey EJW, Overbeek JTG (1948) Theory of the stability of lyophobic colloids. Elsevier Publishing Company, Inc, Amsterdam
Wakindiki I, Ben-Hur M (2002) Soil mineralogy and texture effects on crust micromorphology, infiltration, and erosion. Soil Sci Soc Am J 66:897–905
Warrington D, Mamedov A, Bhardwaj A, Levy G (2009) Primary particle size distribution of eroded material affected by degree of aggregate slaking and seal development. Eur J Soil Sci 60:84–93
Wuddivira MN, Stone RJ, Ekwue EI (2009) Clay, organic matter, and wetting effects on splash detachment and aggregate breakdown under intense rainfall. Soil Sci Soc Am J 73:226–232
Yang JL, Zhang GL, Shi XZ, Wang HJ, Cao ZH, Ritsema CJ (2009) Dynamic changes of nitrogen and phosphorus losses in ephemeral runoff processes by typical storm events in Sichuan Basin, Southwest China. Soil Tillage Res 105:292–299
Acknowledgments
This work was supported by the Natural Science Foundation Project of CQ CSTC (Grant No. CSTC, 2011BA7001) and 973 Program (Grant No. 2010CB134511), National Natural Science Foundation of China (Grant No. 40671090), and Scientific and Technological Innovation Foundation of Southwest University for Graduates (Grant No. kb2010013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Rongliang Qiu
Rights and permissions
About this article
Cite this article
Xu, CY., Yu, ZH. & Li, H. The coupling effects of electric field and clay mineralogy on clay aggregate stability. J Soils Sediments 15, 1159–1168 (2015). https://doi.org/10.1007/s11368-015-1063-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1063-0