Abstract
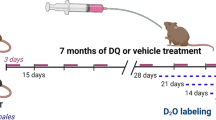
Despite the universal impact of sarcopenia on compromised health and quality of life in the elderly, promising pharmaceutical approaches that can effectively mitigate loss of muscle and function during aging have been limited. Our group and others have reported impairments in peripheral motor neurons and loss of muscle innervation as initiating factors in sarcopenia, contributing to mitochondrial dysfunction and elevated oxidative stress in muscle. We recently reported a reduction in α motor neuron loss in aging mice in response to the compound OKN-007, a proposed antioxidant and anti-inflammatory agent. In the current study, we asked whether OKN-007 treatment in wildtype male mice for 8–9 months beginning at 16 months of age can also protect muscle mass and function. At 25 months of age, we observed a reduction in the loss of whole-body lean mass, a reduced loss of innervation at the neuromuscular junction and well-preserved neuromuscular junction morphology in OKN-007 treated mice versus age matched wildtype untreated mice. The loss in muscle force generation in aging mice (~ 25%) is significantly improved with OKN-007 treatment. In contrast, OKN-007 treatment provided no protection in loss of muscle mass in aging mice. Mitochondrial function was improved by OKN-007 treatment, consistent with its potential antioxidative properties. Together, these exciting findings are the first to demonstrate that interventions through neuroprotection can be an effective therapy to counter aging-related muscle dysfunction.






Similar content being viewed by others
Data availability
The data that support the findings of the study are available in the manuscript and supplementary material of this article. Correspondence and requests for information should be addressed to H.V.R.
Abbreviations
- NMJ:
-
Neuromuscular junction
- ACHR:
-
Acetylcholine receptor
- BSCB:
-
Blood-spinal cord barrier
- OCR:
-
Oxygen consumption rate
- ROS:
-
Reactive oxygen species
- EDL:
-
Extensor digitorum longus
- SERCA:
-
Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase
References
Piekarz KM, et al. Molecular changes associated with spinal cord aging. Geroscience. 2020;42(2):765–84.
Zhou M, et al. Aging process of the human lumbar spinal cord: A morphometric analysis. Neuropathology. 1996;16(2):106–11.
Drey M, et al. Motoneuron loss is associated with sarcopenia. J Am Med Dir Assoc. 2014;15(6):435–9.
Piasecki M, et al. Age-related neuromuscular changes affecting human vastus lateralis. J Physiol. 2016;594(16):4525–36.
Xu H, et al. Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia. J Cachexia Sarcopenia Muscle. 2021;12(6):1582–96.
Bhaskaran S, et al. Neuron-specific deletion of CuZnSOD leads to an advanced sarcopenic phenotype in older mice. Aging Cell. 2020;19(10):e13225.
Sataranatarajan K, et al. Neuron specific reduction in CuZnSOD is not sufficient to initiate a full sarcopenia phenotype. Redox Biol. 2015;5:140–8.
Sakellariou GK, et al. Neuron-specific expression of CuZnSOD prevents the loss of muscle mass and function that occurs in homozygous CuZnSOD-knockout mice. FASEB J. 2014;28(4):1666–81.
Jang YC, et al. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell. 2012;11(5):770–82.
Floyd RA, et al. Nitrone-based therapeutics for neurodegenerative diseases: their use alone or in combination with lanthionines. Free Radic Biol Med. 2013;62:145–56.
Towner RA, et al. OKN-007 Increases temozolomide (TMZ) sensitivity and suppresses TMZ-resistant glioblastoma (GBM) tumor growth. Transl Oncol. 2019;12(2):320–35.
Fan LW, et al. Interleukin-1 beta-Induced brain injury and neurobehavioral dysfunctions in juvenile rats can be attenuated by alpha-Phenyl-N-Tert-Butyl-Nitrone. Neuroscience. 2010;168(1):240–52.
Coutinho de Souza P, et al. OKN-007 decreases free radical levels in a preclinical F98 rat glioma model. Free Radic Biol Med. 2015;87:157–68.
Thomas L, et al. OKlahoma Nitrone-007: novel treatment for diffuse intrinsic pontine glioma. J Transl Med. 2020;18(1):424.
Clausen F, et al. The nitrone free radical scavenger NXY-059 is neuroprotective when administered after traumatic brain injury in the rat. J Neurotrauma. 2008;25(12):1449–57.
Culot M, et al. Cerebrovascular protection as a possible mechanism for the protective effects of NXY-059 in preclinical models: An in vitro study. Brain Res. 2009;1294:144–52.
Floyd R, Hensley K, Bing G. Evidence for enhanced neuro-inflammatory processes in neurodegenerative diseases and the action of nitrones as potential therapeutics. Adv Res Neurodegen. 2000; 387–414.
Piekarz KM, et al. Pharmacologic treatment with OKN-007 reduces alpha-motor neuron loss in spinal cord of aging mice. Geroscience. 2022;44(1):67–81.
Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci U S A. 1997;94(21):11168–72.
Battiste JD, et al. Phase Ib clinical trial of OKN-007 in recurrent malignant glioma. J Clin Oncol. 2020;38(15):2538.
Xu H, et al. Age related changes in muscle mass and force generation in the triple transgenic (3xTgAD) mouse model of alzheimer’s disease. Front Aging Neurosci. 2022;14:876816.
Xu H, Ahn B, Van Remmen H. Impact of aging and oxidative stress on specific components of excitation contraction coupling in regulating force generation. Sci Adv. 2022;8(43):eadd7377.
Xu H, et al. Modulation of sarcopenia phenotypes by glutathione peroxidase 4 overexpression in mice. J Physiol. 2023;601(23):5277–93.
Ahn B, et al. Scavenging mitochondrial hydrogen peroxide by peroxiredoxin 3 overexpression attenuates contractile dysfunction and muscle atrophy in a murine model of accelerated sarcopenia. Aging Cell. 2022;21(3):e13569.
Qaisar R, et al. Restoration of sarcoplasmic reticulum Ca2+ ATPase (SERCA) activity prevents age-related muscle atrophy and weakness in mice. Int J Mol Sci. 2021;22(1):37.
Krumschnabel G, et al. Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Methods Mol Biol. 2015;1264:245–61.
Vasilaki A, et al. The effect of lengthening contractions on neuromuscular junction structure in adult and old mice. Age (Dordr). 2016;38(4):259–72.
Pratt SJP, et al. Recovery of altered neuromuscular junction morphology and muscle function in mdx mice after injury. Cell Mol Life Sci. 2015;72(1):153–64.
Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012;2012:194821.
Muller FL, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40(11):1993–2004.
Larsson L, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2019;99(1):427–511.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–37.
Barreiro E, et al. Both oxidative and nitrosative stress are associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2005;579(7):1646–52.
Chugh D, et al. Neuromuscular junction transmission failure is a late phenotype in aging mice. Neurobiol Aging. 2020;86:182–90.
Anagnostou ME, Hepple RT. Mitochondrial mechanisms of neuromuscular junction degeneration with aging. Cells. 2020;9(1):197.
Pollock N, et al. Neuromuscular junction structure and redox status of skeletal muscle fiber: effect on schwann cells. Faseb J. 2019;33(S1):593.4.
Deepa SS, et al. Accelerated sarcopenia in Cu/Zn superoxide dismutase knockout mice. Free Radic Biol Med. 2019;132:19–23.
Li ZW, et al. Blocking the EGFR/p38/NF-kappaB signaling pathway alleviates disruption of BSCB and subsequent inflammation after spinal cord injury. Neurochem Int. 2021;150:105190.
Tang RZ, et al. The Inhibition of inflammatory signaling pathway by secretory leukocyte protease inhibitor can improve spinal cord injury. Cell Mol Neurobiol. 2020;40(7):1067–73.
Zhang YQ, et al. CuZnSOD gene deletion targeted to skeletal muscle leads to loss of contractile force but does not cause muscle atrophy in adult mice. FASEB J. 2013;27(9):3536–48.
Jang YC, et al. Increased superoxide accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24(5):1376–90.
Bravo-Sagua R, et al. Calcium transport and signaling in mitochondria. Compr Physiol. 2017;7(2):623–34.
Xu H, Van Remmen H. The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: a potential target for intervention in aging and skeletal muscle pathologies. Skelet Muscle. 2021;11(1):25.
Funding
This work was supported by the VA pilot grant (I21 BX005619), and P01 grant (P01 AG051442) from NIA, and also the VA Senior Research Career Scientist award (IK6 BX005234) to Dr. Holly Van Remmen. Dr. Jacob L. Brown is currently supported by a VA Career Development Award (1 IK2 BX005620-01A1).
Author information
Authors and Affiliations
Contributions
H.X., K.M.P., S.B., J.L.B. and H.V.R. contributed in the conception and design of the research; H.X., K.M.P., S.B., J.L.B. performed the experiments and analyzed the data; H.X., K.M.P., S.B., J.L.B. and H.V.R. interpreted the results of the experiments; H.X. and H.V.R. prepared the figures and drafted the manuscript; H.X., S.B., J.L.B. and H.V.R. edited and revised the manuscript. All authors approved the final version of the manuscript, contributed to the article, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Xu, H., Piekarz, K.M., Brown, J.L. et al. Neuroprotective treatment with the nitrone compound OKN-007 mitigates age-related muscle weakness in aging mice. GeroScience (2024). https://doi.org/10.1007/s11357-024-01134-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01134-y