Abstract
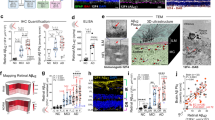
Alzheimer’s disease (AD) exerts a tremendous socio-economic burden worldwide. Although reduced cerebral blood flow is an early and persistent symptom that precedes the loss of cognitive function in AD, the underlying molecular and cellular mechanisms remain unclear. The present study investigated whether capillary endothelial inward rectifier potassium 2 (Kir2.1) expression is reduced in TgF344-AD (AD) rats and contributes to neurovascular uncoupling and cognitive deficits in AD. Three- to fourteen-month-old AD rats expressing mutant human APP and PS1 and age-matched wild-type (WT) F344 rats were studied. AD rats exhibited higher amyloid beta (Aβ) expression in the brain as early as 3 months of age and amyloid plaques by 4 months of age. Functional hyperemic responses induced by whisker stimulation were impaired at 4 months of age, which were exacerbated in 6-month- and 14-month-old AD rats. The expression of Kir2.1 protein was significantly lower in the brains of 6-month-old AD versus WT rats, and Kir2.1 coverage was lower in the cerebral microvasculature of AD than in WT rats. Aβ1–42 reduced the Kir2.1 expression in cultured capillary endothelial cells. Cerebral parenchymal arterioles with attached capillaries exhibited a reduced vasodilator in response to 10 mM K+ applied to capillaries, and constricted less following administration of a Kir2.1 channel blocker, compared to WT vessels. These results indicate that capillary endothelial Kir2.1 expression is reduced and contributes to impaired functional hyperemia in AD rats at early ages, perhaps secondary to elevated Aβ expression.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Gaugler J, James B, Johnson T, Reimer J, Solis M, Weuve J. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18:700–89. https://doi.org/10.1002/alz.12638.
Dhapola R, Hota SS, Sarma P, Bhattacharyya A, Medhi B, Reddy DH. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology. 2021;29:1669–81. https://doi.org/10.1007/s10787-021-00889-6.
Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. https://doi.org/10.1038/ncomms11934.
Korte N, Nortley R, Attwell D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020;140:793–810. https://doi.org/10.1007/s00401-020-02215-w.
Bracko O, Cruz Hernández JC, Park L, Nishimura N, Schaffer CB. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer’s disease. J Cereb Blood Flow Metab. 2021;41:1501–16. https://doi.org/10.1177/0271678x20982383.
Fang X, Zhang J, Roman RJ, Fan F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? GeroScience. 2022;44:1879–83. https://doi.org/10.1007/s11357-022-00591-7.
Fang X, Crumpler RF, Thomas KN, Mazique JN, Roman RJ and Fan F. Contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia. Physiol Int. 2022. https://doi.org/10.1556/2060.2022.00020.
Tarantini S, Fulop GA, Kiss T, Farkas E, Zölei-Szénási D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–73. https://doi.org/10.1007/s11357-017-9980-z.
Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–40. https://doi.org/10.1073/pnas.97.17.9735.
Li L, Tong XK, Hosseini Kahnouei M, Vallerand D, Hamel E, Girouard H. Impaired hippocampal neurovascular coupling in a mouse model of Alzheimer’s disease. Front Physiol. 2021;12:715446. https://doi.org/10.3389/fphys.2021.715446.
Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–60. https://doi.org/10.1038/nrn1387.
Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J Neurosci. 2012;32:4705–15. https://doi.org/10.1523/jneurosci.0169-12.2012.
Joo IL, Lam WW, Oakden W, Hill ME, Koletar MM, Morrone CD, Stanisz GJ, McLaurin J, Stefanovic B. Early alterations in brain glucose metabolism and vascular function in a transgenic rat model of Alzheimer’s disease. Prog Neurobiol. 2022;217:102327. https://doi.org/10.1016/j.pneurobio.2022.102327.
Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–8. https://doi.org/10.1126/science.3616619.
Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–6. https://doi.org/10.1073/pnas.0914722107.
Straub AC, Zeigler AC, Isakson BE. The myoendothelial junction: connections that deliver the message. Physiology (Bethesda). 2014;29:242–9. https://doi.org/10.1152/physiol.00042.2013.
Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–26. https://doi.org/10.1038/nn.4533.
Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation. 2015;22:183–96. https://doi.org/10.1111/micc.12190.
Harraz OF, Longden TA, Dabertrand F, Hill-Eubanks D, Nelson MT. Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc Natl Acad Sci U S A. 2018;115:E3569-e3577. https://doi.org/10.1073/pnas.1800201115.
Moshkforoush A, Ashenagar B, Harraz OF, Dabertrand F, Longden TA, Nelson MT, Tsoukias NM. The capillary Kir channel as sensor and amplifier of neuronal signals: modeling insights on K+-mediated neurovascular communication. Proc Natl Acad Sci U S A. 2020;117:16626–37. https://doi.org/10.1073/pnas.2000151117.
Sackheim AM, Villalba N, Sancho M, Harraz OF, Bonev AD, D'Alessandro A, Nemkov T, Nelson MT and Freeman K. Traumatic brain injury impairs systemic vascular function through disruption of inward-rectifier potassium channels. Function (Oxf). 2021;2. https://doi.org/10.1093/function/zqab018.
Lacalle-Aurioles M, Trigiani LJ, Bourourou M, Lecrux C, Hamel E. Alzheimer’s disease and cerebrovascular pathology alter inward rectifier potassium (KIR 2.1) channels in endothelium of mouse cerebral arteries. Br J Pharmacol. 2022;179:2259–74. https://doi.org/10.1111/bph.15751.
Taylor JL, Pritchard HAT, Walsh KR, Strangward P, White C, Hill-Eubanks D, Alakrawi M, Hennig GW, Allan SM, Nelson MT, Greenstein AS. Functionally linked potassium channel activity in cerebral endothelial and smooth muscle cells is compromised in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2022;119:e2204581119. https://doi.org/10.1073/pnas.2204581119.
Mughal A, Harraz OF, Gonzales AL, Hill-Eubanks D, Nelson MT. PIP2 improves cerebral blood flow in a mouse model of Alzheimer’s disease. Function (Oxf). 2021;2:zqab010. https://doi.org/10.1093/function/zqab010.
Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. https://doi.org/10.1152/physrev.00021.2009.
Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci. 2013;33:6245–56. https://doi.org/10.1523/JNEUROSCI.3672-12.2013.
Fang X, Tang C, Zhang H, Border JJ, Liu Y, Shin SM, Yu H, Roman RJ, Fan F. Longitudinal characterization of cerebral hemodynamics in the TgF344-AD rat model of Alzheimer’s disease. Geroscience. 2023. https://doi.org/10.1007/s11357-023-00773-x.
Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–94. https://doi.org/10.1007/s10571-007-9195-4.
Ruck T, Bittner S, Epping L, Herrmann AM and Meuth SG. Isolation of primary murine brain microvascular endothelial cells. J Vis Exp. 2014:e52204. https://doi.org/10.3791/52204.
Damisah EC, Hill RA, Tong L, Murray KN, Grutzendler J. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat Neurosci. 2017;20:1023–32. https://doi.org/10.1038/nn.4564.
Liu Y, Zhang H, Wu CY, Yu T, Fang X, Ryu JJ, Zheng B, Chen Z, Roman RJ, Fan F. 20-HETE-promoted cerebral blood flow autoregulation is associated with enhanced pericyte contractility. Prostaglandins Other Lipid Mediat. 2021;154:106548. https://doi.org/10.1016/j.prostaglandins.2021.106548.
Wang S, Lv W, Zhang H, Liu Y, Li L, Jefferson JR, Guo Y, Li M, Gao W, Fang X. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. GeroScience. 2020;42:1387–410.
Wang S, Zhang H, Liu Y, Li L, Guo Y, Jiao F, Fang X, Jefferson JR, Li M, Gao W, Gonzalez-Fernandez E, Maranon RO, Pabbidi MR, Liu R, Alexander BT, Roman RJ, Fan F. Sex differences in the structure and function of rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2020;318:H1219–32. https://doi.org/10.1152/ajpheart.00722.2019.
Fan F, Geurts AM, Murphy SR, Pabbidi MR, Jacob HJ, Roman RJ. Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt-sensitive rat. Am J Physiol Regul Integr Comp Physiol. 2015;308:R379–90. https://doi.org/10.1152/ajpregu.00256.2014.
Fan F, Geurts AM, Pabbidi MR, Smith SV, Harder DR, Jacob H, Roman RJ. Zinc-finger nuclease knockout of dual-specificity protein phosphatase-5 enhances the myogenic response and autoregulation of cerebral blood flow in FHH.1BN rats. PLoS One. 2014;9:e112878. https://doi.org/10.1371/journal.pone.0112878.
Guo Y, Wang S, Liu Y, Fan L, Booz GW, Roman RJ, Chen Z, Fan F. Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction. Geroscience. 2020;42:547–61. https://doi.org/10.1007/s11357-020-00179-z.
Liu Y, Zhang H, Wang S, Guo Y, Fang X, Zheng B, Gao W, Yu H, Chen Z, Roman RJ, Fan F. Reduced pericyte and tight junction coverage in old diabetic rats are associated with hyperglycemia-induced cerebrovascular pericyte dysfunction. Am J Physiol Heart Circ Physiol. 2021;320:H549-h562. https://doi.org/10.1152/ajpheart.00726.2020.
Wang S, Lv W, Zhang H, Liu Y, Li L, Jefferson JR, Guo Y, Li M, Gao W, Fang X, Paul IA, Rajkowska G, Shaffery JP, Mosley TH, Hu X, Liu R, Wang Y, Yu H, Roman RJ, Fan F. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience. 2020;42:1387–410. https://doi.org/10.1007/s11357-020-00233-w.
Liu Y, Zhang H, Wu CYC, Yu T, Fang X, Ryu JJ, Zheng B, Chen Z. Roman RJ and Fan F. 20-HETE-promoted cerebral blood flow autoregulation is associated with enhanced pericyte contractility. Prostaglandins Other Lipid Mediat. 2021;154:106548. https://doi.org/10.1016/j.prostaglandins.2021.106548.
Zhang H, Zhang C, Liu Y, Gao W, Wang S, Fang X, Guo Y, Li M, Liu R, Roman RJ, Sun P, Fan F. Influence of dual-specificity protein phosphatase 5 on mechanical properties of rat cerebral and renal arterioles. Physiol Rep. 2020;8:e14345. https://doi.org/10.14814/phy2.14345.
Wang S, Jiao F, Border JJ, Fang X, Crumpler RF, Liu Y, Zhang H, Jefferson J, Guo Y, Elliott PS, Thomas KN, Strong LB, Urvina AH, Zheng B, Rijal A, Smith SV, Yu H, Roman RJ, Fan F. Luseogliflozin, a sodium-glucose cotransporter-2 inhibitor, reverses cerebrovascular dysfunction and cognitive impairments in 18-mo-old diabetic animals. Am J Physiol Heart Circ Physiol. 2022;322:H246–59. https://doi.org/10.1152/ajpheart.00438.2021.
Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab. 2013;33:1486–92. https://doi.org/10.1038/jcbfm.2013.99.
Rosehart AC, Johnson AC, Dabertrand F. Ex vivo pressurized hippocampal capillary-parenchymal arteriole preparation for functional study. J Vis Exp. 2019. https://doi.org/10.3791/60676.
Golding EM, Steenberg ML, Johnson TD, Bryan RM Jr. The effects of potassium on the rat middle cerebral artery. Brain Res. 2000;880:159–66. https://doi.org/10.1016/s0006-8993(00)02793-1.
Youmans KL, Tai LM, Kanekiyo T, Stine WB Jr, Michon SC, Nwabuisi-Heath E, Manelli AM, Fu Y, Riordan S, Eimer WA, Binder L, Bu G, Yu C, Hartley DM, LaDu MJ. Intraneuronal Aβ detection in 5xFAD mice by a new Aβ-specific antibody. Mol Neurodegener. 2012;7:8. https://doi.org/10.1186/1750-1326-7-8.
Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer’s disease. J Biomed Sci. 2020;27:18. https://doi.org/10.1186/s12929-019-0609-7.
Anckaerts C, Blockx I, Summer P, Michael J, Hamaide J, Kreutzer C, Boutin H, Couillard-Després S, Verhoye M, Van der Linden A. Early functional connectivity deficits and progressive microstructural alterations in the TgF344-AD rat model of Alzheimer’s disease: a longitudinal MRI study. Neurobiol Dis. 2019;124:93–107. https://doi.org/10.1016/j.nbd.2018.11.010.
Rorabaugh JM, Chalermpalanupap T, Botz-Zapp CA, Fu VM, Lembeck NA, Cohen RM, Weinshenker D. Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain. 2017;140:3023–38. https://doi.org/10.1093/brain/awx232.
Bernaud VE, Bulen HL, Peña VL, Koebele SV, Northup-Smith SN, Manzo AA, Valenzuela Sanchez M, Opachich Z, Ruhland AM, Bimonte-Nelson HA. Task-dependent learning and memory deficits in the TgF344-AD rat model of Alzheimer’s disease: three key timepoints through middle-age in females. Sci Rep. 2022;12:14596. https://doi.org/10.1038/s41598-022-18415-1.
Joo IL, Lai AY, Bazzigaluppi P, Koletar MM, Dorr A, Brown ME, Thomason LA, Sled JG, McLaurin J, Stefanovic B. Early neurovascular dysfunction in a transgenic rat model of Alzheimer’s disease. Sci Rep. 2017;7:46427. https://doi.org/10.1038/srep46427.
Fang X, Zhang H, Border JJ, Rivers PL, Strong LB, Cooper J, Crumpler RF, Thomas KN, Roman RJ and Fan F. Contribution of beta-amyloid accumulation to cerebral hypoperfusion in Alzheimer’s disease. FASEB J. 2022;36. https://doi.org/10.1096/fasebj.2022.36.S1.R2710.
Fang X, Zhang H, Wang S, Liu Y, Gao W, Roman RJ, Fan F. Abstract P076: cerebral vascular dysfunction precedes cognitive impairment in Alzheimer’s disease. Hypertension. 2020;76:AP076–AP076. https://doi.org/10.1161/hyp.76.suppl_1.P076.
Apátiga-Pérez R, Soto-Rojas LO, Campa-Córdoba BB, Luna-Viramontes NI, Cuevas E, Villanueva-Fierro I, Ontiveros-Torres MA, Bravo-Muñoz M, Flores-Rodríguez P, Garcés-Ramirez L, de la Cruz F, Montiel-Sosa JF, Pacheco-Herrero M, Luna-Muñoz J. Neurovascular dysfunction and vascular amyloid accumulation as early events in Alzheimer’s disease. Metab Brain Dis. 2022;37:39–50. https://doi.org/10.1007/s11011-021-00814-4.
Canobbio I, Abubaker AA, Visconte C, Torti M, Pula G. Role of amyloid peptides in vascular dysfunction and platelet dysregulation in Alzheimer’s disease. Front Cell Neurosci. 2015;9:65. https://doi.org/10.3389/fncel.2015.00065.
Stavljenic-Rukavina A. Molecular mechanisms in Alzheimer’s disease. eJIFCC. 2004;15:100–3.
Bishay J, Beckett TL, Lai AY, Hill ME, McMahon D, McLaurin J. Venular amyloid accumulation in transgenic Fischer 344 Alzheimer’s disease rats. Sci Rep. 2022;12:15287. https://doi.org/10.1038/s41598-022-19549-y.
Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer’s disease - one peptide, two pathways. Nat Rev Neurol. 2020;16:30–42. https://doi.org/10.1038/s41582-019-0281-2.
Attems J, Lauda F, Jellinger KA. Unexpectedly low prevalence of intracerebral hemorrhages in sporadic cerebral amyloid angiopathy: an autopsy study. J Neurol. 2008;255:70–6. https://doi.org/10.1007/s00415-008-0674-4.
Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–5. https://doi.org/10.1073/pnas.1011321108.
Fowler CF, Goerzen D, Devenyi GA, Madularu D, Chakravarty MM, Near J. Neurochemical and cognitive changes precede structural abnormalities in the TgF344-AD rat model. Brain Commun. 2022;4:fcac072. https://doi.org/10.1093/braincomms/fcac072.
Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ, Jacobs RE, Doubal FN, Ramirez J, Black SE, Nedergaard M, Benveniste H, Dichgans M, Iadecola C, Love S, Bath PM, Markus HS, Al-Shahi Salman R, Allan SM, Quinn TJ, Kalaria RN, Werring DJ, Carare RO, Touyz RM, Williams SCR, Moskowitz MA, Katusic ZS, Lutz SE, Lazarov O, Minshall RD, Rehman J, Davis TP, Wellington CL, González HM, Yuan C, Lockhart SN, Hughes TM, Chen CLH, Sachdev P, O’Brien JT, Skoog I, Pantoni L, Gustafson DR, Biessels GJ, Wallin A, Smith EE, Mok V, Wong A, Passmore P, Barkof F, Muller M, Breteler MMB, Román GC, Hamel E, Seshadri S, Gottesman RF, van Buchem MA, Arvanitakis Z, Schneider JA, Drewes LR, Hachinski V, Finch CE, Toga AW, Wardlaw JM, Zlokovic BV. Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15:158–67. https://doi.org/10.1016/j.jalz.2018.07.222.
Wang S, Tang C, Liu Y, Border JJ, Roman RJ, Fan F. Impact of impaired cerebral blood flow autoregulation on cognitive impairment. Front Aging. 2022;3:1077302. https://doi.org/10.3389/fragi.2022.1077302.
Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–31. https://doi.org/10.1038/s41593-018-0234-x.
Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–8. https://doi.org/10.1016/j.exger.2016.11.004.
Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–44. https://doi.org/10.1111/acel.12372.
Tarantini S, Nyúl-Tóth Á, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, Ungvari Z, Csiszar A. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43:2387–94. https://doi.org/10.1007/s11357-021-00405-2.
Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–81. https://doi.org/10.1038/jcbfm.2015.162.
Harraz OF, Hill-Eubanks D, Nelson MT. PIP2: a critical regulator of vascular ion channels hiding in plain sight. Proc Natl Acad Sci U S A. 2020;117:20378–89. https://doi.org/10.1073/pnas.2006737117.
Hakim MA, Behringer EJ. Development of Alzheimer’s disease progressively alters sex-dependent KCa and sex-independent KIR channel function in cerebrovascular endothelium. J Alzheimers Dis. 2020;76:1423–42. https://doi.org/10.3233/jad-200085.
Iadecola C. Cerebrovascular effects of amyloid-beta peptides: mechanisms and implications for Alzheimer’s dementia. Cell Mol Neurobiol. 2003;23:681–9. https://doi.org/10.1023/A:1025092617651.
Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A. 2007;104:365–70. https://doi.org/10.1073/pnas.0609551104.
Behringer EJ, Hakim MA, Blackwell J and Pires PW. Endothelial KIR channel dysfunction in aged cerebral parenchymal arterioles. FASEB J. 2022;36. https://doi.org/10.1096/fasebj.2022.36.S1.R5757.
Okamoto H, Meng W, Ma J, Ayata C, Roman Richard J, Bosnjak Zeljko J, Kampine John P, Huang Paul L, Moskowitz Michael A, Hudetz AG. Isoflurane-induced cerebral hyperemia in neuronal nitric oxide synthase gene deficient mice. Anesthesiology. 1997;86:875–84. https://doi.org/10.1097/00000542-199704000-00018.
van der Schoor L, van Hattum EJ, de Wilde SM, Harlianto NI, van Weert AJ, Bloothooft M and van der Heyden MAG. Towards the development of AgoKirs: new pharmacological activators to study K ir 2.x channel and target cardiac disease. Int J Mol Sci. 2020;21. https://doi.org/10.3390/ijms21165746.
Sancho M and Welsh DG. Chapter eight - KIR channels in the microvasculature: regulatory properties and the lipid-hemodynamic environment. In: Jackson WF, ed. Current topics in membranes. Academic Press; 2020;85: 227–259.
Li J, Lü S, Liu Y, Pang C, Chen Y, Zhang S, Yu H, Long M, Zhang H, Logothetis DE, Zhan Y, An H. Identification of the Conformational transition pathway in PIP2 Opening Kir Channels. Sci Rep. 2015;5:11289. https://doi.org/10.1038/srep11289.
Dabertrand F, Harraz OF, Koide M, Longden TA, Rosehart AC, Hill-Eubanks DC, Joutel A and Nelson MT. PIP2 corrects cerebral blood flow deficits in small vessel disease by rescuing capillary Kir2.1 activity. Proc Natl Acad Sci U S A. 2021;118. https://doi.org/10.1073/pnas.2025998118.
Funding
This study was supported by grants R01 AG057842, RF1 AG079336, and HL138685 from the National Institutes of Health; 23PRE1018124 from the American Heart Association; and G20221001-3551 from Sigma Xi.
Author information
Authors and Affiliations
Contributions
F. F., X. F., and R. J. R. conceived and designed the research project; X. F., J. B., P. R., and H. Z. performed the experiments; X. F., J. B., P. R., R. J. R., and F. F. analyzed the data; X. F., R. J. R., and F. F. interpreted the results of experiments; X. F., H. Z., and F. F. prepared the figures; X. F. and R. J. R. drafted, edited, and revised the manuscript; X. F., F. F., J. B., P. R., H. Z., R. J. R., and J. M. W. approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Fang, X., Border, J.J., Rivers, P.L. et al. Amyloid beta accumulation in TgF344-AD rats is associated with reduced cerebral capillary endothelial Kir2.1 expression and neurovascular uncoupling. GeroScience 45, 2909–2926 (2023). https://doi.org/10.1007/s11357-023-00841-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00841-2