Abstract
Ivermectin, an antiparasitic drug, has been repurposed for COVID-19 treatment during the SARS-CoV-2 pandemic. Although its antiviral efficacy was confirmed early in vitro and in preclinical studies, its clinical efficacy remained ambiguous. Our purpose was to assess the efficacy of ivermectin in terms of time to viral clearance based on the meta-analysis of available clinical trials at the closing date of the data search period, one year after the start of the pandemic. This meta-analysis was reported by following the PRISMA guidelines and by using the PICO format for formulating the question. The study protocol was registered on PROSPERO. Embase, MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), bioRvix, and medRvix were searched for human studies of patients receiving ivermectin therapy with control groups. No language or publication status restrictions were applied. The search ended on 1/31/2021 exactly one year after WHO declared the public health emergency on novel coronavirus. The meta-analysis of three trials involving 382 patients revealed that the mean time to viral clearance was 5.74 days shorter in case of ivermectin treatment compared to the control groups [WMD = −5.74, 95% CI (−11.1, −0.39), p = 0.036]. Ivermectin has significantly reduced the time to viral clearance in mild to moderate COVID-19 diseases compared to control groups. However, more eligible studies are needed for analysis to increase the quality of evidence of ivermectin use in COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
COVID-19 disease caused by the novel coronavirus SARS-CoV-2 has rapidly spread worldwide since December 2019, evoking the most devastating pandemic in the twenty-first century. In the first pandemic period, prevention was limited to social distancing and other measures (e.g., wearing a mask, strict hygienic regulations, social distancing). In contrast, the pharmacotherapy of infected patients was relied upon off-label use of some medicines. So far, more than 440 medications have been tried out in the treatment of COVID-19; however, their efficacy is not supported by unequivocal clinical evidence [1]. One of the most widely used and studied drug is ivermectin which is registered to treat lymphatic filariasis, onchocerciasis, and several other parasitic and viral diseases [2].
Several publications support the potential therapeutic use of ivermectin at the molecular and cellular level in COVID-19. Based on a docking study, ivermectin was supposed to be capable of decelerating the viral spread within the human body by binding SARS-CoV-2 and its ACE2 receptor on target cell at two binding sites in the extracellular phase [at position 91 (leucine) of virus spike protein and at position 378 (histidine) of ACE2 molecule] [3]. The prevention of infection may be related to other activities as well as Wagstaff et al. confirmed that ivermectin inhibits the function of importin α/β (Imp α/β) [4]. Moreover, an in silico study revealed that ivermectin, along with other macrocyclic lactone drugs, can block the RNA-dependent RNA polymerase (RdRP) function by preventing the attachment of the RNA template to the enzyme and can also block the RNA elongation by forming H-bonds with two amino acids (Cys622 and Asp760) [5]. Furthermore, ivermectin can dock on the viral-specific RNA helicase, by which action the virus can form only biologically inactive virions [6]. Ivermectin dimers act as ionophores and can transport antiviral zinc ions through the membranes into the cytoplasm from both the intercellular space and the zinc reservoir of the endoplasmic reticulum [7]. In the late phase of COVID-19 disease, ivermectin is able to block the SARS-CoV-2–induced STAT3-mediated cytokine storm [8]. Ivermectin has been shown to exert wide immunomodulatory actions in mouse models and also in humans, by affecting the nicotinic acetylcholine receptor subtype 7 as reported by Laing et al. [9]. Another positive immunomodulatory effect on increased Teff/Treg ratio and tumor targeting CD4+ and CD8+ T cell infiltration was reported in breast cancer mouse model based on interactions with the ATP-P2X4-P2X7 purinergic receptor axis [10]. In children continuously treated for 14 days with a dose of 1000 μg/kg [11] as part of a mixed chemotherapy salvage regimen of acute myeloid leukemia, no toxic effects but stable disease or clinical remission were recorded.
In preclinical in vivo animal studies, ivermectin decreased viral load and improved symptoms in a mouse coronavirus infection with the mouse hepatitis virus (MHV-I) [12]. A preclinical study of ivermectin in a hamster model of SARS-CoV-2 infection [13] proved that ivermectin attenuated clinical scores and symptoms, as well as lung inflammation at 4 days postinfection, after a single subcutaneous dose of 400 μg/kg administered at infection. The symptom-attenuating effects were largely related to the interferon I-III axis, cholinergic and glutamatergic neurotransmitter decrease, and adenylate cyclase gene regulation in this hamster model, somewhat resembling that of corticosteroids and IL-6 antagonists [13].
Based on the robust in vitro antiviral action, the antiviral effects of this compound are under investigation in numerous ongoing studies [14]. However, caution should be taken when translating the in vitro activity to therapeutic efficacy. The first in vitro study of ivermectin on SARS-CoV-2–infected cell lines reported that the compound at a concentration of 5 μM inhibited viral replication within 48 h [15]. However, in this experiment, ivermectin was used at a higher concentration than was previously postulated to be achievable by the typical therapeutic dosage of 150–400 μg/kg. Even in five- or tenfold orally applied concentrations, ivermectin has not shown an increase in adverse effects in human pharmacokinetic studies [16]. Long-standing and widespread use of ivermectin to date has been associated only with infrequent and mostly mild adverse events; severe side effects (encephalopathy) were experienced only in patients co-infected with Loa-Loa [17]. It has been suggested that the polymorphism of the MDR-1 transporter might be related to central nervous system toxicity of ivermectin administration [18]. However, to date, 3.7 billion ivermectin tablet intake has been registered in the VigiBase, whereas only two casualties have been reported as severe neurological adverse events leading to death [18]. Moreover, a recent meta-analysis showed no difference in the severity of the adverse events between standard (up to 400 μg/kg) and higher doses of ivermectin [17].
The number of clinical trials assessing the efficacy of ivermectin in COVID-19 is relatively high [18], while the use of ivermectin has not been accepted in official international organization guidelines, although it is in widespread official use in thirteen countries in Africa, Asia, and Latin-America, with altogether forty-one countries adopting its use to a certain degree in COVID-19 therapy. The European Medicines Agency and the US Food and Drug Administration advised for the use of ivermectin in randomized clinical trials [19, 20]. According to the statement of MSD (the originator of ivermectin) early in the pandemic, there had been no meaningful evidence for the clinical efficacy of ivermectin in patients with COVID-19 disease [21].
Human studies with ivermectin are heterogeneous. Early clinical trials involved diverse study populations treated in different phases of the disease. Defined daily doses, treatment durations, and endpoints are not or just hardly comparable in these trials. In the beginning of the pandemic, efficacy of ivermectin could be assessed based on only case series and retrospective studies; later, randomized controlled trials have been launched.
Our purpose was to evaluate the efficacy of ivermectin in terms of time to viral clearance based on the meta-analysis of available clinical trials in the early period of a year between 30/1/2020 and 31/1/2021.
Materials and methods
The meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA—http://prisma-statement.org/PRISMAStatement/PRISMAStatement.aspx) reporting guidance, and it was registered in the International Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42021253185).
The following PICO (patients, intervention, comparison, outcome) format was applied: P: PCR confirmed COVID-19 infected patients; I: ivermectin alone or in combination with standard care or in combination with other drugs; C: standard care or therapy without ivermectin; and O: days required for viral clearance.
For the purpose of continued information of the field, we also include a scoping review activity of ivermectin-related publications on clinical trial results up to 31 Oct 2022, when the formalization of our manuscript was finished. Study reports were retrieved in English in this case, using ClinicalTrials.gov, Google Scholar, and PubMed and searching for ivermectin application reports in clinical trials with emphasis on relevance to our original PICO: early application, mild-moderate disease, and viral clearance. We include the results of this overview outside the formal results reporting, in Table 4. in the “Discussion” part.
Information sources and search strategy
The systematic literature search included in our meta-analysis was conducted until 31 January, 2021, in Embase, MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), bioRvix, and medRvix by using the following search terms: ((“covid 19”) OR (“Wuhan virus”) OR (“coronavirus”) OR (“2019 nCoV”) OR (“SARS-CoV-2”)) AND (ivermectin). To increase the yield of relevant articles, ClinicalTrials.gov was also searched using the above search terms [(ivermectin | ((“covid 19”) OR (“Wuhan virus”) OR (“coronavirus”) OR (“2019 nCoV”) OR (“SARS-CoV-2”))]. No language, publication date, or publication status restrictions were applied. The reference lists of all identified articles were inspected. Only publicly available data were analyzed; the authors were not contacted for additional information.
Eligibility criteria and study selection
Controlled trials evaluating the effects of ivermectin in PCR confirmed COVID-19 patients were included. Abstracts, case series, case reports, and uncontrolled studies not reporting numerical data on efficacy were excluded. For reference management, EndNote 20 was used. After removing duplicates, the remaining records were screened for eligibility based on their titles and then abstracts. The eligibility of the full texts of the resulting records was assessed by two reviewer teams independently. Based on the agreements and disagreements of the selection, Cohen’s Kappas were calculated. Disagreements between reviewer teams were dissolved by discussion, and if needed, a reviewer previously not involved in the selection was consulted.
Data extraction and synthesis of results
Study characteristics and results were searched by the two review authors independently. The following data items were extracted from the included papers: study design, characteristics of the patient population and sample size, intervention details, type of comparator(s), outcome measures, and overall results. Days required for viral clearance were extracted as primary outcome measure. Discrepancies in extracted data were resolved by discussion between the two review authors.
Risk of bias
The risk of bias of controlled randomized studies was analyzed using the Cochrane Collaboration tool for which includes the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other scores of bias. For each domain, studies were judged to have a high (red), unclear (yellow), or low (green) risk of bias (see Supplementary Figs S1and S2). Disagreement was resolved by discussion. Risk of bias figures were prepared by using the RevMan 5 statistical program [22].
The risk of bias in non-randomized studies of interventions (ROBINS-I) tool was used for assessing the risk of bias of the non-randomized interventional studies [23]. Seven different domains were assessed: confounding, selection of participants, classifications of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported outcome. In the end, an overall bias assessment was performed. After evaluation, low, moderate, high risk of bias, or no information were indicated with green, yellow, red, and gray, respectively (see Supplementary FigS3).
The two authors (B.T. and F.D.) first assessed the risk of bias within the selected studies independently, and disagreements were resolved by a third investigator (M.M.). Results of the risk of bias assessment were discussed when the limitations of the individual studies were assessed.
Statistical analyses
For data synthesis, the methods recommended by the working group of the Cochrane Collaboration were used [22]. The extracted data allowed us to perform a meta-analysis, and the calculated effect sizes were visualized in a forest plot.
For binary outcomes, odds ratios (OR) were calculated with 95% confidence intervals. For continuous outcomes, weighted mean differences (WMD) or standardized mean differences (SMD) with 95% confidence intervals were calculated to investigate the differences between the two groups (ivermectin group vs. control group).
A random-effects model of DerSimonian and Laird was used. Heterogeneity was assessed by using Cochrane’s Q and the I2 statistics. Based on Cochrane’s handbook, I2 = 100% × (Q−df)/Q represents the magnitude of the heterogeneity (moderate: 30–60%, substantial: 50–90%, considerable: 75–100%). For the meta-analysis, Stata 15 (StataCorp) was used.
Quality of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used for estimating the quality of evidence of all outcomes assessed [24].
Results
Systematic search and study selection
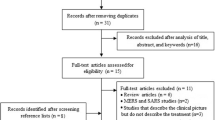
Using the search key ((“covid 19”) OR (“Wuhan virus”) OR (“coronavirus”) OR (“2019 nCoV”) OR (“SARS-CoV-2”) AND (ivermectin) in Embase, MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), bioRvix, and medRvix and removing duplicate results, the search yielded a total of 446 potentially relevant records. The clinical trials included in the meta-analysis were selected according to the PRISMA flow chart presented below (Fig. 1).
PRISMA flow diagram for identification of relevant studies: 322 records that only reported in vitro or animal experiments, or were review papers were excluded. After screening titles and abstracts, 6 publications were retrieved for qualitative synthesis [25,26,27,28,29,30], of which three clinical trials were included in the quantitative analysis [27,28,29] (Table 1)
Qualitative analysis of excluded trials
In total, six studies were selected for the qualitative analysis. Of these six studies, three trials were finally not included in the statistical analysis since these trials did not report the outcome of our predefined PICO.
Camprubi et al. compared 13 COVID-19 patients treated with ivermectin (200 μg/kg, single dose) to 13 COVID-19 patients who were not treated with ivermectin in a retrospective study (n = 26). No difference was found in any reported clinical and microbiological outcomes (PCR positivity 3–5 days after therapy, clinical improvement 8 days after therapy) [25].
Chaccour et al. assessed the efficacy of a single dose of ivermectin (400 μg/kg) in reducing transmission of SARS-CoV-2 when administered early after disease onset in a placebo-controlled, double-blind RCT (n = 24). The primary outcome measure was the proportion of PCR-negative patients at day 7 post-treatment. The viral load and infectivity of each sample were also determined. On day 7, there was no difference in the proportion of PCR positive patients, and the ivermectin group had non-statistically non-significantly lower viral loads at day 4. However, patients in the ivermectin group recovered earlier from hyposmia/anosmia [26].
In these 2 trials, the viral clearance had not been predefined as study outcome.
Okumus et al. conducted an RCT where 66 patients with severe COVID-19 pneumonia were randomized to receive ivermectin (200 μg/kg/day, for 5 days) and a reference treatment protocol hydroxychloroquine, favipiravir, and azithromycin. The control group received only the reference treatment protocol. This study found that adding ivermectin to standard therapy might benefit patients suffering from severe COVID-19 disease (e.g., number of participants with clinical response, oxygen saturation) [30]. In this study, the COVID-19 patients were in severe and not mild or moderate conditions as expected by our PICO.
Quantitative analysis
Two randomized, controlled, and retrospective studies were included in the meta-analysis.
Babalola et al. conducted a randomized, double blind controlled study involving RT-PCR–proven COVID-19 positive patients in Nigeria. Sixty-two patients were randomized to 6-mg or 12-mg ivermectin regime (given every 84 h for 2 weeks) or lopinavir/ritonavir plus standard care. The period needed for viral negativity was significantly shorter in the two ivermectin groups (6.0 ± 2.9 and for 4.6 ± 3.2 days, respectively, and 5.34 ± 0.07 days for the pooled ivermectin group) versus the control group (9.1 ± 5.2 days). Ivermectin treatment also increased platelet count compared to control, and there was a tendency for increased SPO2% in the ivermectin group; however, this difference was not significant. There were no significant changes in hepatic and renal functions, and no adverse drug events were reported spontaneously or in response to inquiry [27].
Ahmed et al. carried out a randomized, double-blind, placebo-controlled trial to determine the duration of viral clearance and safety of ivermectin among adult SARS-CoV-2 patients in Bangladesh. Seventy-two hospitalized patients with mild-moderate disease were assigned to receive ivermectin alone (12 mg once daily for 5 days), or ivermectin in combination with doxycycline (12-mg ivermectin single dose and 200-mg doxycycline on day 1, followed by 100 mg every 12 h for the next 4 days) or placebo. The mean duration to viral clearance was shorter, 9.7 days in the only ivermectin arm (p = 0.02), 11.5 days in the ivermectin + doxycycline (p = 0.27) arm than in the placebo group (12.7 days). In case of remission of fever, cough, and sore throat, there were no differences between the groups. No adverse effects were recorded during the study [28].
Khan et al. conducted a retrospective study on the data of 325 consecutive patients with SARS-CoV-2 infection in Bangladesh, 115 of whom received ivermectin (single 12-mg tablet within 24 h after hospital admission) plus standard of care (SOC), while 133 received only SOC. The groups were compared in terms of time to SARS-CoV-2 negativity, disease progression, duration of hospital stays, and mortality rate. The time to viral negativity in patients treated with ivermectin was shorter than that in the control group (median 4 vs. 15 days; p < 0.001), and the length of hospital stay was also shorter (median 9 vs. 15 days; p < 0.001). A total of 9.8% patients developed pneumonia and 1.5% had ischemic stroke in the control group with no such case in the ivermectin-treated group. Significantly, fewer ivermectin-treated patients required oxygen inhalation (9.6% vs. 45.9), developed respiratory distress (2.6% vs. 15.8%), or needed antibiotic therapy (15.7% vs. 60.2%) or intensive care treatment (0.9% vs. 8.3%). There were no side effects reported that can be related to ivermectin use [29].
Based on the meta-analysis of the results published in the three analyzed studies [27,28,29] (Table 2), the mean time to viral clearance was 5.74 days shorter in the case of patients treated with ivermectin than in the controls [p = 0.036, WMD = −5.74, 95% CI (−11.1, −0.39)] (Fig. 2).
Risk of bias assessment
Overall, the quality of the randomized trials [27, 28] included in our final quantitative analysis was reckoned to be more disquieting than acceptable, mostly with an unclear risk of bias (see Supplementary Figs S1 and S2).
Both randomized studies showed an unclear risk of selection bias because the authors failed to describe the methods used for randomization in detail [27, 28]. Based on the blinding of the personnel and participants and making the interventions as identical as possible, both studies mentioned above had an unclear risk of performance bias. It was not said in either of the studies whether the intervention and the comparator were identical in size, shape, color, and odor. Furthermore, the authors failed to describe precisely who exactly was blinded, and it was not mentioned in either of the studies whether unblinding occurred before or after data analysis, and the outcome assessment was performed in a blinded manner or not; hence, both studies have an unclear risk of detection bias. Ahmed et al. failed to report on every outcome included in the methods section; therefore, their study was judged to have an unclear risk of reporting bias. The study of Babalola et al. had a low risk of reporting bias, and both randomized controlled studies showed a low risk of attrition bias [27, 28].
The study of Ahmed et al. had an unclear risk of other bias since one of the sponsors manufactures ivermectin-based medication, and it was not indicated whether the sponsor had any influence on the design or the execution of the study [28]. Babalola et al. did not mention any sponsors and conflicts of interest; nevertheless, this does not mean that there were none; therefore, we are uncertain that this study has a low risk of other bias; hence, it was judged as unclear [27].
Khan et al. conducted a retrospective study, and after assessing the risk of bias of the study with the ROBINS-I tool, the overall risk of bias was judged to be low [29]. Moderate bias was assumed only in the case of outcome measurements because assessors might have been aware of the intervention received by study participants. The study showed a low risk of bias in all the six remaining domains (see Supplementary FigS3).
Due to the low number of studies, the presence of publication bias could not be assessed by Egger’s test or funnel plots.
Grade of evidence
The grade of evidence of our statements was assessed with the GRADE approach (Table 3). To assess the grade of evidence, we considered five downgrading items (i.e., limitations in the design and implementation, indirectness, heterogeneity, imprecision, and publication bias).
Publication bias is suspected since published evidence includes only a few small trials. Moreover, because of the broad CIs in cases of the trials reported by Babalola et al. and Ahmed et al., imprecision is suspected, and its indirectness is also assumed; hence, the involved patient populations were not homogeneous, and the simultaneously applied therapies are not fully described in the articles [27, 28]. Overall, the finding that ivermectin reduces the time required for viral clearance in COVID-19 patients is supported by very low-quality evidence; i.e., any estimate of effect is very uncertain.
Discussion
In the light of our findings in this review, it is intriguing to note the quite low doses and short duration of treatment in the referenced clinical studies. To better exploit any putative antiviral effect of ivermectin in planning later studies, first, we propose a re-evaluation of achievable tissue-level virucidal ivermectin concentrations. A dose-escalating phase I study had found no evidence of any harm [14] and a wide safety margin, for per os administration in a dose range from 200 to 2000 μg/kg, three times a week. In the same study, it was also shown that peroral ivermectin administration with a high-fat meal increases the maximal plasma concentration of the same dose more than threefold than in fasted subjects. From the reported data in [14], it can be inferred that even a mg/l plasma concentration is achievable using, e.g., a single 120-mg oral dose with a meal. The effect of meal and even beer to increase ivermectin plasma concentrations have also been reported by others, as well as the absorption-increasing effect of fluid formulation [17]. Schmith et al, in their re-estimation of ivermectin pharmacokinetic tissue distribution models, have called for the re-evaluation of posology in COVID studies because they found that by just using a sufficiently long period of daily, fasted administration with 200 μg/kg, one-fourth of the IC50 reported by Caly et al might even be reached in lung tissue [18]. In the same publication, it was shown that tissue concentrations of ivermectin are expected to be 2–4 times higher than the plasma concentrations. Hence, longer-duration dosing in a daily repeat regimen with a meal and at least using double the approved dose could in fact lead to tissue concentrations in the range that have been proven virucidal in vitro. Current clinical and pharmacologic evidence suggests a presumable lack of serious adverse effects from such dose regimens.
Our meta-analysis revealed that the mean time to viral clearance was 5.74 days shorter in patients treated with ivermectin than in those in the control groups. This effect is statistically significant and clinically relevant; however, it should be noted that this result is based on only three clinical studies [27,28,29].
The therapeutic efficacy of ivermectin has been assessed in several clinical trials [25, 30] and meta-analyses. A meta-analysis assessed the therapeutic potential of ivermectin as an add-on treatment [31], whereas one study focused on its potential role in prophylaxis [32]. A meta-analysis (literature search ended on April 9, 2021) analyzed the effect of ivermectin compared to standard of care or placebo on mortality reported as risk ratio (RR). The results of nine randomized, controlled trials (1788 patients) were meta-analyzed. Ivermectin treatment was associated with decreased mortality (RR 0.39, [95% 0.20–0.74], p = 0.004; I2: 58.2%); this effect was not significant in patients with severe COVID-19 (RR 0.42, p = 0.052). The major limitation of this paper was that most of the included studies were preprints. Moreover, in the case of several trials, the sample size was inadequate, and the dosage of ivermectin and the therapy applied in the control group (chloroquine, hydroxychloroquine, favipiravir, standard of care, placebo) was heterogeneous [33].
The primary outcome for the intervention component of the meta-analysis of Bryant et al. included death from any cause and presence of COVID-19 infection. Altogether, 15 trials were (n = 2438) meta-analyzed and it was found that ivermectin reduced risk of death compared with no ivermectin (RR 0.38, [95% 0.19–0.73]; I2 = 49%). The effect was more pronounced in patients with mild to moderate COVID-19 than in severe cases (RR 0.24 vs. 0.51). The limitations of this study are the variability of recruited participants and control treatment regimens [34].
According to a meta-analysis of data obtained involving critically ill patients hospitalized in the intensive care unit (ICU), ivermectin use was associated with lower mortality (OR 0.15, [95% 0.04–0.57]; p = 0.005). However, this result was based only on two trials [35].
Besides the scientific papers reporting meta-analyses, the authors of the webpage www.c19early.com are regularly updating the page with clinical data related to ivermectin use since the outbreak of SARS-CoV-2. As of 8/8/2021, all studies show 86%/74%/43% efficacy in prophylaxis/early treatment/late treatment in 61 trials with 23.309 patients.
The strength of our meta-analysis is that we have followed the most recent guidelines during data collection and analysis. One of the limitations of this strategy is the very limited number of eligible studies for meta-analysis over a relatively short period of time. Two of these were carried out in the same country, and the overall number of involved patients is rather low. Moreover, the dosages of ivermectin, the study durations, and patient populations were heterogeneous and the risk of bias was usually unknown due to the poor reporting expectation. Although our study supports the efficacy of ivermectin in decreasing time to viral clearance, further trials and meta-analyses should be carried out to assess its clinical efficacy. In order to maintain actuality, we also considered providing the reader with the recapitulation of studies published up to October 2022 in a tabular format. These trials [35] are summarized in Table 4. Our findings hint that for further meta-analyses dosage of the trial drug, as well as treatment duration must be accounted for and if possible, dissected further with appropriate statistical methodology. It seems apparent from Table 4 that an increased daily dose of ivermectin and longer time of administration might be offering more therapeutic benefits over the maximally reported 5 days of use. It is interesting to note that recent reports such as Kerr’s [45] can direct towards a possible prophylactic use of ivermectin in public health settings as well.
Conclusions
Ivermectin has significantly reduced the time to viral clearance in mild to moderate COVID-19 diseases compared to control groups. The mean time to viral clearance was 5.74 days shorter in case of patients treated with ivermectin, which may be a therapeutic advantage. However, the quality of evidence is very low and the results of this meta-analysis do not confirm the therapeutic value of ivermectin in terms of symptom relief, decreased risk of hospitalization, or mortality. Several studies have been finished since our closure date and many are still ongoing to reveal the efficacy of ivermectin in COVID-19 disease which we could not include due to conceptual and outcome analysis differences. New research is needed to analyze recent data and to also evaluate the clinical advantage of ivermectin therapy in SARS-CoV-2 infection.
References
Venkatesan P. Repurposing drugs for treatment of COVID-19. Lancet Respir Med. 2021;9(7):e63. https://doi.org/10.1016/S2213-2600(21)00270-8.
Õmura S, Crump A. The life and times of ivermectin—a success story. Nat Rev Microbiol. 2004;2(12):984–9.
Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. in vivo. 2020;34(5):3023–6.
Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443(3):851–6.
Janabi AHD. Effective anti-SARS-CoV-2 RNA dependent RNA polymerase drugs based on docking methods: the case of milbemycin, ivermectin, and baloxavir marboxil. Avicenna J Med Biotechnol. 2020;12(4):246.
Sen Gupta PS, Biswal S, Panda SK, Ray AK, Rana MK. Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with in-vitro effective drug ivermectin. J Biomol Struct Dyn. 2022;40(5):2217–26.
Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(7):1153–6.
Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW. An aberrant STAT pathway is central to COVID-19. Cell Death & Diff. 2020;27(12):3209–25.
Laing R, Gillan V, Devaney E. Ivermectin - old drug, new tricks? Trends Parasitol. 2017;33(6):463–72. https://doi.org/10.1016/j.pt.2017.02.004.
Draganov D, Han Z, Rana A, Bennett N, Irvine DJ, Lee PP. Ivermectin converts cold tumors hot and synergizes with immune checkpoint blockade for treatment of breast cancer. NPJ Breast Cancer. 2021;7(1):22. https://doi.org/10.1038/s41523-021-00229-5.
de Castro CG, Jr., Gregianin LJ, Burger JA. Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection. Leuk Lymphoma. 2020;61(10):2536–7. https://doi.org/10.1080/10428194.2020.1786559.
Arevalo AP, Pagotto R, Pórfido JL, Daghero H, Segovia M, Yamasaki K, et al. Ivermectin reduces in vivo coronavirus infection in a mouse experimental model. Scientific Rep. 2021;11(1):1-12.
de Melo GD, Lazarini F, Larrous F, Feige L, Kornobis E, Levallois S, et al. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol Med. 2021;13(8):e14122. https://doi.org/10.15252/emmm.202114122.
Jans DA, Wagstaff KM. Ivermectin as a broad-spectrum host-directed antiviral: the real deal? Cells. 2020;9(9):2100.
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral res. 2020;178:104787.
Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. The J of Clinical Pharma. 2002;42(10):1122–33.
Navarro M, Camprubí D, Requena-Méndez A, Buonfrate D, Giorli G, Kamgno J, et al. Safety of high-dose ivermectin: a systematic review and meta-analysis. J Antimicrob Chemother. 2020;75(4):827–34.
Chandler RE. Serious neurological adverse events after ivermectin—do they occur beyond the indication of onchocerciasis? Am J Trop Med Hyg. 2018;98(2):382.
EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials. https://www.ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-treatment-COVID-19-outside-randomised-clinical-trials. (2021). Accessed.
Why you should not use ivermectin to treat or prevent COVID-19. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-COVID-19 (2021). Accessed.
Merck statement on ivermectin use during the COVID-19 pandemic. https://www.merck.com/news/merck-statement-on-ivermectin-use-during-the-COVID-19-pandemic/. (2021). Accessed.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2019.
Sterne J, Hernán M, Reeves B, Savović J, Berkman N, Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions BMJ. 2016; 355: i4919. Int J Epidemiol. 2018;1.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–6.
Camprubí D, Almuedo-Riera A, Martí-Soler H, Soriano A, Hurtado JC, Subirà C, et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PloS one. 2020;15(11):e0242184.
Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMed. 2021;32:100720.
Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos. QJM. 2021;114(11):780–8.
Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–6.
Khan MSI, Khan MSI, Debnath CR, Nath PN, Al Mahtab M, Nabeka H, et al. Ivermectin treatment may improve the prognosis of patients with COVID-19. Arch Bronconeumol. 2020;56(12):828.
Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı İY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):1–11.
Padhy BM, Mohanty RR, Das S, Meher BR. Therapeutic potential of ivermectin as add on treatment in COVID 19: a systematic review and meta-analysis: Ivermectin in COVID-19: A meta-analysis. J Pharm Pharm Sci. 2020;23:462–9.
Bartoszko JJ, Siemieniuk RA, Kum E, Qasim A, Zeraatkar D, Ge L, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. bmj. 2021:373.
Zein AFMZ, Sulistiyana CS, Raffaelo WM, Pranata R. Ivermectin and mortality in patients with COVID-19: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Diabetes Metab Syndr. 2021;15(4):102186.
Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;28(4):e434.
Kim MS, An MH, Kim WJ, Hwang T-H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS med. 2020;17(12):e1003501.
Abd-Elsalam S, Noor RA, Badawi R, Khalaf M, Esmail ES, Soliman S, Abd El Ghafar MS, Elbahnasawy M, Moustafa EF, Hassany SM, Medhat MA, Ramadan HK, Eldeen MAS, Alboraie M, Cordie A, Esmat G. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study. J Med Virol. 2021;93(10):5833–5838. https://doi.org/10.1002/jmv.27122.
López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, Díazgranados JA, Oñate JM, Chavarriaga H, Herrera S, Parra B, Libreros G, Jaramillo R, Avendaño AC, Toro DF, Torres M, Lesmes MC, Rios CA, Caicedo I. Effect oilf ivermectin on time to resolution of symptoms among adults with md COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–35. https://doi.org/10.1001/jama.2021.3071.
Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, Campias C, Chaparro Campias E, Medina MF, Achinelli F, Guglielmone HA, Ojeda J, Farizano Salazar D, Andino G, Kawerin P, Dellamea S, Aquino AC, Flores V, Martemucci CN, Martinez SM, Segovia JE, Reynoso PI, Sosa NC, Robledo ME, Guarrochena JM, Vernengo MM, Ruiz Diaz N, Meza E, Aguirre MG. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21(1):635. https://doi.org/10.1186/s12879-021-06348-5.
Mohan A, Tiwari P, Suri TM, Mittal S, Patel A, Jain A, Velpandian T, Das US, Boppana TK, Pandey RM, Shelke SS, Singh AR, Bhatnagar S, Masih S, Mahajan S, Dwivedi T, Sahoo B, Pandit A, Bhopale S, Vig S, Gupta R, Madan K, Hadda V, Gupta N, Garg R, Meena VP, Guleria R. Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial. J Infect Chemother. 2021;27(12):1743–9. https://doi.org/10.1016/j.jiac.2021.08.021.
Samaha AA, Mouawia H, Fawaz M, Hassan H, Salami A, Bazzal AA, Saab HB, Al-Wakeel M, Alsaabi A, Chouman M, Moussawi MA, Ayoub H, Raad A, Hajjeh O, Eid AH, Raad H. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021;13(6):989. https://doi.org/10.3390/v13060989.
Beltran Gonzalez JL, González Gámez M, Mendoza Enciso EA, Esparza Maldonado RJ, Hernández Palacios D, Dueñas Campos S, Robles IO, Macías Guzmán MJ, García Díaz AL, Gutiérrez Peña CM, Martinez Medina L, Monroy Colin VA, Arreola Guerra JM. Efficacy and safety of ivermectin and Hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial. Infect Dis Rep. 2022;14(2):160–8. https://doi.org/10.3390/idr14020020.
Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, et al. Corrigendum to Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial. EClinicalMedicine. 2021;37:100959. https://doi.org/10.1016/j.eclinm.2021.100959.
Biber A, Harmelin G, Lev D, Ram L, Shaham A, Nemet I, et al. The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19 - a double-blind, randomized placebo-controlled trial. Int J Infect Dis. 2022;122:733–40. https://doi.org/10.1016/j.ijid.2022.07.003.
Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Gentile N, Collins S, et al. Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;328(16):1595–603. https://doi.org/10.1001/jama.2022.18590.
Kerr L, Cadegiani FA, Baldi F, Lobo RB, Assagra WLO, Proença FC, et al. Corrected: Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching. Cureus. 2022;14(3):c6. https://doi.org/10.7759/cureus.c61.
Data availability statement
Supporting data can be accessed at the senior author’s workplace, at Institute of Clinical Pharmacy, Faculty of Pharmacy, University of Szeged, Szeged, Hungary.
Funding
Open access funding provided by Semmelweis University. Part of the research leading to these results was funded by HCEMM, a teaming grant associated to the European Molecular Biology Laboratories, from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement no. 739593. DM has received support from the Ministry of Innovation and Technology through Semmelweis University Grant Investment to the Future no. 2020.1.16- JÖVŐ-2021-00013, and the Semmelweis-TKI Physical Virology Research Group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
FigS1 Risk of bias summary: a review of the authors' judgment about each risk of bias item for each included study. FigS2 Risk of bias graph: a review of the authors' judgment about each risk of bias item presented as percentages across all included studies. FigS3 Risk of bias assessment of the study of Khan et al.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ragó, Z., Tóth, B., Szalenko-Tőkés, Á. et al. Results of a systematic review and meta-analysis of early studies on ivermectin in SARS-CoV-2 infection. GeroScience 45, 2179–2193 (2023). https://doi.org/10.1007/s11357-023-00756-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00756-y