Abstract
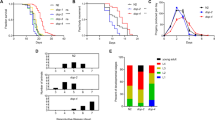
Vitamin D deficiency is associated with a variety of age-related diseases and is becoming increasingly more prevalent in the population over time. Some diseases associated with deficiency are cardiovascular disease, cancer, and neurodegeneration. This association, as well as the fact that vitamin D has been demonstrated to play an important role in a variety of extraskeletal processes, has led some to claim that vitamin D is an essential longevity vitamin. However, the role of vitamin D in healthy aging has been difficult to determine. In order to study vitamin D in the context of aging, the model organism, Caenorhabditis elegans (C. elegans), was employed. To study vitamin D’s impact on aging and age-related disease, lifespan and health span were measured across three different genetic strains of C. elegans. Strains investigated were wildtype (N2), worms with a mutant vitamin D receptor ortholog (nhr-8), and worms engineered to represent Alzheimer disease (gnals2). Bioinformatic analysis of available public data was also performed in order to identify the transcriptional response produced in N2 worms treated with vitamin D3. Treatment with vitamin D3 significantly extended the lifespan of N2 worms and rescued nhr-8 worms, which typically have decreased lifespans compared to N2. Treatment with vitamin D3 minimally extended the lifespan of gnals2 worms. Similar results were obtained for measures of health span, quantified as motility through time. Differentially expressed genes upon treatment with vitamin D3 were largely associated with biological processes such as the innate immune response and metabolism of xenobiotic compounds in the worms, which may explain the observed increase in lifespan and health span.





Similar content being viewed by others
Data availability
Survival and motility data is available via GraphPad Prism data file upon request.
Code availability
All bioinformation analysis was performed in the Galaxy platform at usegalaxy.org. Code used to run analysis was pulled directly from this platform, and associated references are described in the methods.
References
Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US Population, 1988–2004. Arch Intern Med. 2009;169(6):626–32.
Omeed Sizar, Swapnil Khare, Amandeep Goyal, Pankaj Bansal AG. Vitamin D deficiency. Treasure Island, FL: StatPearls Publishing; 2021.Available from: https://www.ncbi.nlm.nih.gov/books/NBK532266/
Tuohimaa P. Vitamin D and aging. J Steroid Biochem Mol Biol. 2009;114(1–2):78–84.
Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, TmavaBerisha A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74(11):1498–513.
Ames BN. Prolonging healthy aging: longevity vitamins and proteins. 2018;115(43). https://doi.org/10.1073/pnas.1809045115
Raed A, Bhagatwala J, Zhu H, Pollock NK, Parikh SJ, Huang Y, et al. Dose responses of vitamin D3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: a placebo controlled randomized trial. PLoS ONE. 2017;12(12):1–13.
Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012;97(8):2670–81.
Guessous I. Role of vitamin D deficiency in extraskeletal complications: predictor of health outcome or marker of health status? Biomed Res Int. 2015;2015. https://doi.org/10.1155/2015/563403
Pilz S, Pilz S, Zittermann A, Trummer C, Theiler-schwetz V, Lerchbaum E, et al. Vitamin D testing and treatment: a narrative review of current evidence. Endocr Connect. 2019;8:27–43.
Jolliffe DA, Walton RT, Griffiths CJ, Martineau AR. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: Review of genetic association studies. J Steroid Biochem Mol Biol. 2016;164:18–29.
Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014;28(6):2398–413.
Olsen A, Vantipalli MC, Lithgow GJ. Using Caenorhabditis elegans as a model for aging and age-related diseases. Ann N Y Acad Sci. 2006;1067(1):120–8.
Zhang S, Li F, Zhou T, Wang G, Li Z. Caenorhabditis elegans as a useful model for studying aging Mutations. Front Endocrinol (Lausanne). 2020;11(October):1–9.
Jones G, Kottler ML, Schlingmann KP. Genetic diseases of vitamin D metabolizing enzymes. Endocrinol Metab Clin North Am. 2017;46(4):1095–117.
Mark KA, Dumas KJ, Bhaumik D, Schilling B, Davis S, Oron TR, et al. Vitamin D promotes protein homeostasis and longevity via the stress response pathway genes skn-1, ire-1, and xbp-1. Cell Rep. 2016;17(5):1227–37.
Bouillon R, Suda T. Vitamin D: calcium and bone homeostasis during evolution. Bonekey Rep. 2013;2014(3):1–10.
Messing J, Heuberger R, Schisa J. Effect of Vitamin D3 on lifespan in Caenorhabditis elegans. Curr Aging Sci. 2014;6(3):220–4.
Leiteritz A, Schmiedl T, Baumanns S, Wenzel U. Amyloid-beta induced paralysis is reduced by cholecalciferol through inhibition of the steroid-signaling pathway in an Alzheimer model of Caenorhabditis elegans. Nutr Neurosci. 2019;0(0):1–8.
Stiernagle T. Maintenance of C. elegans. WormBook. 1999;2006:1–11.
Afgan E, Baker D, van den Beek M, Blankenberg D, Bouvier D, Čech M, et al. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44(W1):W3-10.
Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: Archive for functional genomics data sets - Update. Nucleic acids Res. 2013;41(D1):991–5.
Wingett SW, Andrews S. Fastq screen: A tool for multi-genome mapping and quality control [version 1; referees: 3 approved, 1 approved with reservations]. F1000Research. 2018;7:1–14.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.j. 2011;17(1):10–2.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60.
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):1–21.
Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, et al. G:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47(W1):W191–8.
Magner DB, Wollam J, Shen Y, Hoppe C, Li D, Latza C, et al. The NHR-8 nuclear receptor regulates cholesterol and bile acid homeostasis in C. elegans. Cell Metab. 2013;18(2):212–24.
Son HG, Altintas O, Kim EJE, Kwon S, Lee SJV. Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell. 2019;18(2):1–11.
Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer’s disease and dementia: Evidence from meta-analysis. Nutr J. 2015;14(1):1–5.
Senchuk M, Dues D, Van Raamsdonk J. Measuring oxidative stress in Caenorhabditis elegans: Paraquat and Juglone Sensitivity Assays. Bio-Protoc. 2017;7(1):1–16.
Whitfield GK, Dang HTL, Schluter SF, Bernstein RM, Bunag T, Manzon LA, et al. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinol. 2003;144(6):2704–16.
Hanel A, Carlberg C. Vitamin D and evolution: Pharmacologic implications. Biochem Pharmacol. 2020;173. https://doi.org/10.1016/j.bcp.2019.07.024
Kutuzova GD, DeLuca HF. 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicol Appl Pharmacol. 2007;218(1):37–44.
Gómez-Linton DR, Alavez S, Alarcón-Aguilar A, López-Diazguerrero NE, Konigsberg M, Pérez-Flores LJ. Some naturally occurring compounds that increase longevity and stress resistance in model organisms of aging. Biogerontology. 2019;20(5):583–603.
Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–6.
Acknowledgements
Thank you to Arijit Mukherjee, Ben Cash, Erin Wiley, Mick Yoder, Steve Karafit, Autumn Kennedy, and Julian Stobaugh for equipment use and project assistance.
Funding
Funding provided by the University of Central Arkansas, University Research Council and the College of Natural Sciences and Mathematics Student Research Fund.
Author information
Authors and Affiliations
Contributions
BH performed all experiments. MF supervised the project. BH and MF wrote the manuscript.
Corresponding author
Ethics declarations
Consent for publication
The authors consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Huggins, B., Farris, M. Vitamin D3 promotes longevity in Caenorhabditis elegans. GeroScience 45, 345–358 (2023). https://doi.org/10.1007/s11357-022-00637-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00637-w