Abstract
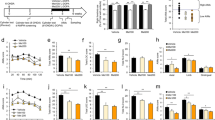
Identifying neurobiological mechanisms of aging-related parkinsonism, and lifestyle interventions that mitigate them, remain critical knowledge gaps. No aging study, from rodent to human, has reported loss of any dopamine (DA) signaling marker near the magnitude associated with onset of parkinsonian signs in Parkinson’s disease (PD). However, in substantia nigra (SN), similar loss of DA signaling markers in PD or aging coincide with parkinsonian signs. Alleviation of these parkinsonian signs may be possible by interventions such as calorie restriction (CR), which augment DA signaling markers like tyrosine hydroxylase (TH) expression in the SN, but not striatum. Here, we interrogated respective contributions of nigral and striatal DA mechanisms to aging-related parkinsonian signs in aging (18 months old) rats in two studies: by the imposition of CR for 6 months, and inhibition of DA uptake within the SN or striatum by cannula-directed infusion of nomifensine. Parkinsonian signs were mitigated within 12 weeks after CR and maintained until 24 months old, commensurate with increased D1 receptor expression in the SN alone, and increased GDNF family receptor, GFR-α1, in the striatum, suggesting increased GDNF signaling. Nomifensine infusion into the SN or striatum selectively increased extracellular DA. However, only nigral infusion increased locomotor activity. These results indicate mechanisms that increase components of DA signaling in the SN alone mitigate parkinsonian signs in aging, and are modifiable by interventions, like CR, to offset parkinsonian signs, even at advanced age. Moreover, these results give evidence that changes in nigral DA signaling may modulate some parameters of locomotor activity autonomously from striatal DA signaling.






Similar content being viewed by others
Data availability
Data and resource sharing are available upon request.
References
Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an aging society. Milbank Q. 2009;87:842–62.
Anderson LA, Goodman RA, Holtzman D, Posner SF, Northridge ME. Aging in the United States: opportunities and challenges for public health. Am J Public Health. 2012;102:393–5.
Avendano M, Glymour MM, Banks J, Mackenbach JP. Health disadvantage in US adults aged 50 to 74 years: a comparison of the health of rich and poor Americans with that of Europeans. Am J Public Health. 2011;99:540–8.
Wahrendorf M, Reinhardt JD, Siegrist J. Relationships of disability with age among adults aged 50 to 85: evidence from the United States. England and Continental Europe Plos One. 2013;8: e71893.
Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, et al. Prevalence of Parkinsonian signs and associated mortality in a community population of older people. New Engl J Medicine. 1996;334:71–6.
Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493–8.
Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, et al. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol. 2004;56:532–9.
Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55:11–9.
Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71:258–66.
Buchman AS, Leurgans SE, Yu L, Wilson RS, Lim AS, James BD, et al. Incident parkinsonism in older adults without Parkinson disease. Neurology. 2016;87:1036–44.
Collier TJ, Kanaan NM, Kordower JH. Aging and Parkinson’s disease: different sides of the same coin? Mov Disord Official J Mov Disord Soc. 2017;32:983–90.
Clark BC, Woods AJ, Clark LA, Criss CR, Shadmehr R, Grooms DR. The aging brain & the dorsal basal ganglia: implications for age-related limitations of mobility. Adv Geriatric Medicine Res. 2019;1: e190008.
Crimmins EM. Lifespan and healthspan: past, present, and promise. Gerontologist. 2015;55:901–11.
Sorond FA, Cruz-Almeida Y, Clark DJ, Viswanathan A, Scherzer CR, Jager PD, et al. Aging, the central nervous system, and mobility in older adults: neural mechanisms of mobility impairment. Journals Gerontology Ser. 2015;70:1526–32.
Varma VR, Hausdorff JM, Studenski SA, Rosano C, Camicioli R, Alexander NB, et al. Aging, the central nervous system, and mobility in older adults: interventions. J Gerontol Ser Biol Sci Med Sci. 2016;71:1451–8.
Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, et al. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–65.
Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in Brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–56.
Zhang Z, Andersen A, Smith C, Grondin R, Gerhardt G, Gash D. Motor slowing and Parkinsonian signs in aging rhesus monkeys mirror human aging. J Gerontol Ser. 2000;55:B473–80.
Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci. 2003;23:1974–80.
Salvatore MF, Pruett BS, Spann SL, Dempsey C. Aging reveals a role for nigral tyrosine hydroxylase ser31 phosphorylation in locomotor activity generation. PLoS ONE. 2009;4: e8466.
Salvatore MF, Terrebonne J, Cantu MA, McInnis TR, Venable K, Kelley P, et al. Dissociation of striatal dopamine and tyrosine hydroxylase expression from aging-related motor decline: evidence from calorie restriction intervention. J Gerontol A Biol Sci. 2017;73:11–20.
Arnold JC, Cantu MA, Kasanga EA, Nejtek VA, Papa EV, Bugnariu N, et al. Aging-related limit of exercise efficacy on motor decline. PLoS ONE. 2017;12: e0188538.
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55.
Marsden CD. Parkinson’s disease. Lancet. 1990;335:948–9.
Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci. 2001;21:6853–61.
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–31.
Kish SJ, Shannak K, Rajput A, Deck JHN, Hornykiewicz O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson’s disease. J Neurochem. 1992;58:642–8.
Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, et al. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65.
Wolf ME, LeWitt PA, Bannon MJ, Dragovic LJ, Kapatos G. Effect of aging on tyrosine hydroxylase protein content and the relative number of dopamine nerve terminals in human caudate. J Neurochem. 1991;56:1191–200.
Irwin I, DeLanney LE, McNeill T, Chan P, Forno LS, Murphy GM, et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegener J Neurodegener Disord Neuroprotection Neuroregener. 1994;3:251–65.
Gerhardt GA, Cass WA, Henson M, Zhang Z, Ovadia A, Hoffer BJ, et al. Age-related changes in potassium-evoked overflow of dopamine in the striatum of the rhesus monkey. Neurobiol Aging. 1995;16:939–46.
Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80:168–77.
Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87:574–85.
Salvatore MF, Terrebonne J, Fields V, Nodurft D, Runfalo C, Latimer B, et al. Initiation of calorie restriction in middle-aged male rats attenuates aging-related motoric decline and bradykinesia without increased striatal dopamine. Neurobiol Aging. 2016;37:192–207.
Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–301.
Salvatore MF, Pruett BS. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritic and terminal field areas of nigrostriatal and mesoaccumbens pathways. PLoS ONE. 2012;7: e29867.
Buchman AS, Dawe RJ, Yu L, Lim A, Wilson RS, Schneider JA, et al. Brain pathology is related to total daily physical activity in older adults. Neurology. 2018;90:e1911–9.
Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–60.
Windels F, Kiyatkin EA. Dopamine action in the substantia nigra pars reticulata: iontophoretic studies in awake, unrestrained rats. Eur J Neurosci. 2006;24:1385–94.
Kliem MA, Maidment NT, Ackerson LC, Chen S, Smith Y, Wichmann T. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol. 2007;98:1489–500.
Zaman V, Boger HA, Granholm A, Rohrer B, Moore A, Buhusi M, et al. The nigrostriatal dopamine system of aging GFRα-1 heterozygous mice: neurochemistry, morphology and behavior. Eur J Neurosci. 2008;28:1557–68.
Trevitt J, Carlson B, Nowend K, Salamone J. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology. 2001;156:32–41.
Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, et al. Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology. 1991;103:41–5.
Salvatore MF, McInnis TR, Cantu MA, Apple DM, Pruett BS. Tyrosine hydroxylase inhibition in substantia nigra decreases movement frequency. Mol Neurobiol. 2019;56:2728–40.
Pruett BS, Salvatore MF. Nigral GFRα1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol Neurobiol. 2013;47:988–99.
Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and Brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15:319–28.
Kasanga EA, Little J, McInnis TR, Bugnariu N, Cunningham JT, Salvatore MF. Cardiovascular metrics associated with prevention of aging-related Parkinsonian signs following exercise intervention in sedentary older rats. Front Aging Neurosci. 2021;13: 775355.
Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Res. 1998;797:42–54.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12.
Spielewoy C, Roubert C, Hamon M, Nosten M, Betancur C, Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol. 2000;11:279–90.
Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered locomotion in the absence of decreased locomotor activity: exacerbation by nomifensine. Neurosci Lett. 2002;333:195–8.
Salvatore MF, Pruett BS, Dempsey C, Fields V. Comprehensive profiling of dopamine regulation in substantia nigra and ventral tegmental area. J Vis Exp. 2012;e4171
Robertson G, Damsma G, Fibiger H. Characterization of dopamine release in the substantia nigra by in vivo microdialysis in freely moving rats. J Neurosci. 1991;11:2209–16.
Kasanga EA, Owens CL, Cantu MA, Richard AD, Davis RW, McDivitt LM, et al. GFR-α1 expression in substantia nigra increases bilaterally following unilateral striatal GDNF in aged rats and attenuates nigral tyrosine hydroxylase loss following 6-OHDA nigrostriatal lesion. ACS Chem Neurosci. 2019;10:4237–49.
Athanasiou KA, Zhu CF, Wang X, Agrawal CM. Effects of aging and dietary restriction on the structural integrity of rat articular cartilage. Ann Biomed Eng. 2000;28:143–9.
Kolta MG, Holson R, Duffy P, Hart RW. Effect of long-term caloric restriction on brain monoamines in aging male and female Fischer 344 rats. Mech Ageing Dev. 1989;48:191–8.
Marshall JF, Rosenstein AJ. Age-related decline in rat striatal dopamine metabolism is regionally homogeneous. Neurobiol Aging. 1990;11:131–7.
Friedemann MN, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13:325–32.
Salvatore MF. ser31 tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J Neurochem. 2014;129:548–58.
Pruett BS, Salvatore MF. GFR α-1 receptor expression in the aging nigrostriatal and mesoaccumbens pathways. J Neurochem. 2010;115:707–15.
Schultz W, Ruffieux A, Aebischer P. The activity of pars compacta neurons of the monkey substantia nigra in relation to motor activation. Exp Brain Res. 1983;51:377–87.
Dodson PD, Dreyer JK, Jennings KA, Syed ECJ, Wade-Martins R, Cragg SJ, et al. Representation of spontaneous movement by dopaminergic neurons is cell-type selective and disrupted in parkinsonism. Proc National Acad Sci. 2016;113:E2180–8.
da Silva JA, Tecuapetla F, Paixão V, Costa RM. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature. 2018;554:244–8.
Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–96.
Dave KD, Silva SD, Sheth NP, Ramboz S, Beck MJ, Quang C, et al. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis. 2014;70:190–203.
Pérez-Taboada I, Alberquilla S, Martín ED, Anand R, Vietti-Michelina S, Tebeka NN, et al. Diabetes causes dysfunctional dopamine neurotransmission favoring nigrostriatal degeneration in mice. Movement Disord. 2020;35:1636–48.
Zhang Y, Meng X, Jiao Z, Liu Y, Zhang X, Qu S. Generation of a novel mouse model of Parkinson’s disease via targeted knockdown of glutamate transporter GLT-1 in the substantia nigra. Acs Chem Neurosci. 2020;11:406–17.
Kordower JH, Goetz CG, Chu Y, Halliday GM, Nicholson DA, Musial TF, et al. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann Neurol. 2017;81:46–57.
Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–5.
Gerhardt GA, Cass WA, Huettl P, Brock S, Zhang Z, Gash DM. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res. 1999;817:163–71.
Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77:378–90.
Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–300.
Bergquist F, Shahabi HN, Nissbrandt H. Somatodendritic dopamine release in rat substantia nigra influences motor performance on the accelerating rod. Brain Res. 2003;973:81–91.
González-Rodríguez P, Zampese E, Stout KA, Guzman JN, Ilijic E, Yang B, et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature. 2021;599:650–6.
von Linstow CU, DeLano-Taylor M, Kordower JH, Brundin P. Does developmental variability in the number of midbrain dopamine neurons affect individual risk for sporadic Parkinson’s disease? J Park Dis. 2020;10:405–11.
McGeer PL, McGeer EG, Suzuki JS. Aging and extrapyramidal function. Arch Neurol-chicago. 1977;34:33–5.
Bayliss JA, Lemus MB, Stark R, Santos VV, Thompson A, Rees DJ, et al. Ghrelin-AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson’s disease. J Neurosci. 2016;36:3049–63.
Lahiri AK, Bevan MD. Dopaminergic transmission rapidly and persistently enhances excitability of D1 receptor-expressing striatal projection neurons. Neuron. 2020;106:277-290.e6.
Gnanalingham KK, Jenner P, Hunter AJ, Marsden CD. Selective dopamine antagonist pretreatment on the antiparkinsonian effects of benzazepine D1 dopamine agonists in rodent and primate models of parkinson’s disease — the differential effects of D1 dopamine antagonists in the primate. Psychopharmacology. 1995;117:403–12.
Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Be. 2002;72:857–63.
Guo M, Xiang T, Li M, Sun Y, Sun S, Chen D, et al. Effects of intrastriatal injection of the dopamine receptor agonist SKF38393 and quinpirole on locomotor behavior in hemiparkinsonism rats. Behav Brain Res. 2021;411: 113339.
Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–67.
Ouyang J, Carcea I, Schiavo JK, Jones KT, Rabinowitsch A, Kolaric R, et al. Food restriction induces synaptic incorporation of calcium-permeable AMPA receptors in nucleus accumbens. Eur J Neurosci. 2017;45:826–36.
Wang Y, Bouabid S, Darvas M, Zhou F-M. The antiparkinson drug ropinirole inhibits movement in a Parkinson’s disease mouse model with residual dopamine neurons. Exp Neurol. 2020;333: 113427.
Bello EP, Casas-Cordero R, Galiñanes GL, Casey E, Belluscio MA, Rodríguez V, et al. Inducible ablation of dopamine D2 receptors in adult mice impairs locomotion, motor skill learning and leads to severe parkinsonism. Mol Psychiatr. 2017;22:595–604.
Diao LH, Bickford PC, Stevens JO, Cline EJ, Gerhardt GA. Caloric restriction enhances evoked DA overflow in striatum and nucleus accumbens of aged Fischer 344 rats. Brain Res. 1997;763:276–80.
Dunn JP, Abumrad NN, Kessler RM, Patterson BW, Li R, Marks-Shulman P, et al. Caloric restriction-induced decreases in dopamine receptor availability are associated with leptin concentration. Obesity. 2017;25:1910–5.
Tomac A, Lindqvist E, Lin L-FH, Ögren SO, Young D, Hoffer BJ, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–9.
Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc National Acad Sci. 2004;101:18171–6.
Suda Y, Kuzumaki N, Narita M, Hamada Y, Shibasaki M, Tanaka K, et al. Effect of ghrelin on the motor deficit caused by the ablation of nigrostriatal dopaminergic cells or the inhibition of striatal dopamine receptors. Biochem Bioph Res Co. 2018;496:1102–8.
Ingram DK, de Cabo R. Calorie restriction in rodents: caveats to consider. Ageing Res Rev. 2017;39:15–28.
Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–7.
Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontology. 1987;42:78–81.
Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557.
Balasubramanian P, Howell PR, Anderson RM. Aging and caloric restriction research: a biological perspective with translational potential. EBioMedicine. 2017;21:37–44.
Yamada Y, Kemnitz JW, Weindruch R, Anderson RM, Schoeller DA, Colman RJ. Caloric restriction and healthy life span: frail phenotype of nonhuman primates in the Wisconsin National Primate Research Center Caloric Restriction Study. J Gerontol Ser. 2017;73:273–8.
Pifferi F, Aujard F. Caloric restriction, longevity and aging: recent contributions from human and non-human primate studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;95: 109702.
Cava E, Fontana L. Will calorie restriction work in humans? Aging Albany Ny. 2013;5:507–14.
Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: an update. Ageing Res Rev. 2017;39:36–45.
Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol Ser. 2015;70:1097–104.
Romashkan SV, Das SK, Villareal DT, Ravussin E, Redman LM, Rochon J, et al. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget. 2016;7:19124–33.
Ingram DK, Roth GS. Glycolytic inhibition: an effective strategy for developing calorie restriction mimetics. Geroscience. 2021;43:1159–69.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (AG040261) to MFS, and the Office of Vice president for Research and Innovation and Institute for Healthy Aging at University of North Texas health Science Center and the National Institute on Aging (AG020494) to EAK.
Author information
Authors and Affiliations
Contributions
Study design: MFS, CB, DKI. Conducted the experiments: EAK, KEV, DPK, TRM, MAC, JT, KL, SMM, AC. Analyzed the data: KEV, DPK, EAK, MFS, CB. Wrote the manuscript: MFS, EAK, KEV, DPK. Reviewed the manuscript: CB, DKI. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Salvatore, M.F., Kasanga, E.A., Kelley, D.P. et al. Modulation of nigral dopamine signaling mitigates parkinsonian signs of aging: evidence from intervention with calorie restriction or inhibition of dopamine uptake. GeroScience 45, 45–63 (2023). https://doi.org/10.1007/s11357-022-00583-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00583-7