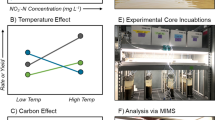
Abstract
An increase in water temperature is one of the main factors that can potentially modify biogeochemical dynamics in lowland rivers, such as the removal and recycling of nitrogen (N). This effect of climate change on N processing deserves attention, as it may have unexpected impacts on eutrophication in the coastal zones. Intact sediment cores were collected seasonally at the closing section of the Po River, the largest Italian river and one of the main N inputs to the Mediterranean Sea. Benthic oxygen fluxes, denitrification, and dissimilatory nitrate reduction to ammonium (DNRA) rates were measured using laboratory dark incubations. Different temperature treatments were set up for each season based on historical data and future predictions. Higher water temperatures enhanced sediment oxygen demand and the extent of hypoxic conditions in the benthic compartment, favoring anaerobic metabolism. Indeed, warming water temperature stimulated nitrate (NO3−) reduction processes, although NO3− and organic matter availability were found to be the main controlling factors shaping the rates between seasons. Denitrification was the main process responsible for NO3− removal, mainly supported by NO3− diffusion from the water column into the sediments, and much more important than N recycling via DNRA. The predicted increase in the water temperature of the Po River due to climate change may exert an unexpected negative feedback on eutrophication by strongly controlling denitrification and contributing to partial buffering of N export in the lagoons and coastal areas, especially in spring.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers are heavily affected by anthropogenic activities, particularly by nitrogen (N) input from agricultural sources, mostly in the form of nitrate ion (NO3−) (Lassaletta et al. 2009; Sutton et al. 2011). Nitrogen loads can affect the trophic state of rivers and, when delivered to terminal water bodies, fuel eutrophication and trigger algal blooms in both transitional and coastal zones (Dodds 2006; Glibert 2017). The generation of N loads, their transport across land-river and river-sea interfaces, and eutrophication dynamics are strongly influenced by land use, river morphology, and hydrological and thermal conditions, all of which are subject to climate change (Hou et al. 2019; Romero et al. 2013; Tu 2009).
Extreme hydrological events such as floods and droughts, which have increased in frequency in recent years, directly impact river discharge and the generation and delivery of nutrient loads to water bodies, transport dynamics within the hydrological network, and microbial processes of N transformation and removal (Abily et al. 2021; Zheng et al. 2023). River sediments are active sites for biogeochemical reactions (Triska and Higler 2009), such as denitrification and DNRA. The denitrification is the anaerobic respiration that converts NO3− to di-nitrogen gas (N2), the final product, and nitrite (NO2−) and nitrous oxide (N2O), as intermediate products. It is the most important process supporting the self-depuration capacity of rivers, removing NO3− permanently from the ecosystem (Hill 2023; Piña-Ochoa and Álvarez-Cobelas 2006; Seitzinger 1988). In contrast, the dissimilatory nitrate reduction to ammonium (DNRA) is an alternative microbial pathway for NO3− reduction, using the same substrates as denitrification (NO3− and organic carbon), but recycling N in the ecosystem through the conversion of NO3− to ammonium (NH4+) (Giblin et al. 2013).
River denitrification is controlled by several factors, such as oxygen concentration at the water-sediment interface, and the availability of NO3− and labile organic carbon (Ballard et al. 2019; Hu et al. 2023; Piña-Ochoa and Álvarez-Cobelas 2006). One of the most important environmental drivers influencing denitrification is water temperature (de Klein et al. 2017; Veraart et al. 2011). Warming boosts denitrification alongside enzymatic reactions, but water temperature increase also has an impact on the process by regulating two of the aforementioned determinants of denitrification, i.e., oxygen concentration and availability of labile organic matter (de Klein et al. 2017; Speir et al. 2023). Indeed, all biogeochemical NO3− dissimilatory pathways are affected by water warming, both as a direct effect of temperature on enzyme activity and an indirect effect on sediment redox conditions (Brin et al. 2017). Furthermore, the availability of labile organic carbon, oxygen, and redox conditions in sediments determine the relative roles of denitrification and DNRA in NO3− removal or recycling in aquatic ecosystems (Nizzoli et al. 2010; Aalto et al. 2021).
Under climate change scenarios, a reduction in rainfall, runoff, and river flow is likely to lower NO3− concentrations (Oduor et al. 2023), thereby limiting river denitrification. On the other hand, the expected increase in water temperature is likely to stimulate the process. Several studies have isolated the effect of temperature on denitrification through manipulative experiments (e.g., Silvennoinen et al. 2008; Velthuis and Veraart 2022; Speir et al. 2023). Nevertheless, there is a lack of systematic research predicting the consequences of warming on the self-depuration capacity of large lowland rivers, given their crucial role in processing anthropogenic N inputs along the land-sea continuum.
The Po is the largest Italian river in terms of watershed area and annual discharge at the closing section and is one of the major rivers in the Mediterranean region (Struglia et al. 2004). Its catchment is one of the most industrialized and intensively farmed catchments in the world (Moatti and Thiébault 2016), making it a hotspot for NO3− pollution. The Po River contributes two-thirds of the total freshwater discharge and nutrient inputs conveyed to the Adriatic Sea (Grilli et al. 2020; Viaroli et al. 2018) and is the major basin for riverine N export to the Mediterranean Sea (Romero et al. 2021). In the last decades, the Po River basin was strongly affected by the increasing frequency of extreme events due to climate change (Appiotti et al. 2014; Coppola et al. 2014; Marchina et al. 2017). For example, in 2022, rainfall and river discharge were the lowest in historical records since 1961 (Montanari et al. 2023) and water temperature was the highest measured in the last two decades (Gervasio et al. 2023). Following these extreme hydrological and thermal conditions, experimental work was carried out on intact sediment cores sampled from the lower course of the Po River to measure benthic denitrification and DNRA rates. The aim of the present study was to assess the seasonal effect of temperature and NO3− availability on the river buffering capacity against N pollution and eutrophication in the coastal zone.
Materials and methods
Study area
The Po River is the longest and most important Italian river, flowing from the Alps to the Adriatic Sea, with an average annual discharge of 1500 m3 s−1 at the Pontelagoscuro (Ferrara, 44°53′16.9″N, 11°36′26.6″E) measuring station, the official basin closing section (Fig. 1; Zanchettin et al. 2008). The basin covers an area of approximately 71,000 km2 across Italy, a quarter of the national territory. The river has more than 140 tributaries and a capillary network of artificial irrigation and drainage canals (Soana et al. 2019). The basin is subject to a mix of subcontinental and warm-temperature climates (the Mediterranean climate), which split the annual hydrological regime into two low-flow periods (winter and summer) and two recharging periods (spring and autumn) fed by snowmelt and rainfall (Coppola et al. 2014; Montanari 2012; Ravazzani et al. 2015). In recent decades, the Po River basin has experienced the effects of climate change, with an increase in extreme storm events (Brunetti et al. 2004; Domeneghetti et al. 2015; Giambastiani et al. 2017), long drought periods, and water temperature warming (Gervasio et al. 2022; Bonaldo et al. 2023; Soana et al. 2023). The Po River crosses the Po Valley, the most fertile and extensively cultivated area in Italy, and is the main source of irrigation water for crops. Since the 1960s, the intensification of agriculture and livestock farming has made the Po River the main source of nutrient inputs to the North Adriatic Sea, triggering algal blooms during warm periods (Penna et al. 2004; Spillman et al. 2007).
Map of the Po River course (blue line) and its basin (bordered with the red line and grey area on top) located in Northern Italy (modified by ArcGIS 10.8.2, ESRI). The red star indicates the sampling station in Pontelagoscuro. The black dots indicate the monitoring temperature stations belonging to the Regional Agency for Environmental Prevention of Emilia – Romagna, Lombardy, and Veneto Regions: Sermide (S 1), Stellata - Bondeno (S 2), Polesella (S 3), and Serravalle (S 4), situated in the province of Ferrara (Italy)
Sampling activities
Sediment cores were sampled in the Po River at Pontelagoscuro (red star in Fig. 1), about 90 km from the main mouth to the Adriatic Sea, in winter (February), spring (May), summer (July), and autumn (November). Water column parameters (temperature, electrical conductivity, and oxygen concentration) were measured in situ using a multiparametric probe (YSI Model 85-Handheld Dissolved Oxygen, Conductivity, Salinity and Temperature System, Yellow Springs, OH, USA) during the sampling days. Sampling, pre-incubation, and incubation were performed according to standardized protocols (Dalsgaard 2000). The experimental design consisted of 25 intact sediment cores (plexiglass liners, internal diameter 4.5 cm, length 20 cm) collected for each seasonal sampling with a hand corner from the boat. The cores were sampled at depths ranging from 1.5 to 4 m, according to the different seasonal hydrological conditions of the Po River. All cores were leveled in order to have a sediment height of about 9 cm and an overlying water column of about 8 cm. After collection, intact sediment cores were submerged in tanks with site water continuously aerated using aquarium pumps and transported to the laboratory. Approximately 60 L of bottom water was collected and brought to the laboratory for core maintenance, pre-incubation, and incubation procedure. Five of the intact sediment cores were randomly assigned to each of the four temperature treatments and incubated in the dark to determine oxygen fluxes, denitrification, and DNRA rates. The remaining five cores were used for sediment characterization.
Seasonal gradients of water temperature
During each seasonal incubation, the temperature range was established from the historical temperature data of the Po River (1992–2022), monitored monthly by the Regional Environmental Protection Agencies of the Emilia – Romagna, Lombardy, and Veneto Regions (named ARPAE, ARPA of the Lombardy Region, and ARPAV, respectively) in five sections along a lowland 80-km stretch right upstream of the deltaic system: Sermide, (S1), Stellata–Bondeno (S2), Pontelagoscuro, our sediment sampling site, Polesella (S3) and Serravalle (S4) (black dots in Fig. 1). The Po River water temperature has gradual increased over the last decades (1992–2022; Fig. 2) and the upward trend was particularly marked in summer and autumn (nearly +5 °C; Fig. 2). Summer average temperature ranged from 22 (1996) to 27 °C (2022), showing a significant upward trend equivalent to a raise of 0.15 °C yr-1. In autumn, the trend was similar in slope to summer (0.16 °C yr-1), with values varying between 10 (1998) and 15 °C (2014). Although the average winter and spring temperature trends were not statistically significant, a slight increase was observed, with values ranging from 7 to 10 °C and from 17 to 20 °C, respectively. Extreme temperatures, both minimum and maximum, have also increased since the 1990s with a few exceptions. The minimum winter temperatures ranged from 3 to 7 °C, while spring values varied between 10 and 15 °C. Finally, summer minimum temperatures ranged from 15 to 23 °C, while the autumn minimum temperatures did not increase significantly. Maximum temperatures, on the other hand, did not show a significant trend, but there were some years with maximum temperatures higher than usual, such as 1998 for spring and autumn and the early 2000s for all seasons.
Historical average, minimum, and maximum water temperatures (°C) of the Lower Po River since the 1990s in each season, monitoring from Sermide to Serravalle sections. a Winter: January – March period. b Spring: April – June period. c Summer: July – September period. d Autumn: October – December. Solid lines show statistically significant trends
Based on historical water temperatures time series, four different temperatures were applied in each seasonal incubation, covering the following ranges: 5–14 °C in winter (January, February, March), 13–22 °C in spring (April, May, June), 21–30 °C in summer (July, August, September), and 9–18 °C in autumn (October, November, December) (Table 1). The lower extreme of each seasonal range corresponded to the average of the seasonal minimum temperature measured in the Po River from the 1990s to the present, while the upper extreme of the range was established based on the maximum values expected in the near future owing to climate warming. In the period 2041–2070, air temperatures are expected to increase throughout the Po basin in all seasons, with positive anomalies of up to 3 °C (Vezzoli et al. 2015). Intermediate temperatures correspond to the most frequent seasonal mean temperatures over the last 30 years. The experimental temperature in the incubation tanks was controlled using a thermostat (Fig. 3b) and continuously monitored during each incubation using a multi-parameter probe.
Seasonal incubation procedure
Each core was equipped with a rotating Teflon-coated magnet driven by an external magnet connected to a motor (40 rpm). Inside the cores, the magnet was suspended a few centimeters above the sediment-water interface to gently mix the water column while avoiding resuspension (Fig. 3a). To allow for acclimatization, each target temperature level was set in the early afternoon of the day before the start of incubation. According to standardized protocols (Dalsgaard 2000; Owens and Cornwell 2016), intact sediment cores were incubated in batch mode to measure benthic dark oxygen fluxes (sediment oxygen demand, SOD). Dark incubations were chosen to simulate in situ conditions, where light penetration is limited by turbidity and the benthic compartment is in the dark (Braga et al. 2017). The water in each tank was replaced to maintain in situ dissolved nutrient concentrations. Before the incubation of the benthic fluxes, O2 was measured using a multiparametric probe inside each core. The water level in the tanks was lowered to a few centimeters below the top of the cores, and each liner was sealed with a plexiglass lid (Fig. 3a). Incubation times ranged from 1.5 (summer experiment) to 4 (winter experiment) hours and were set, based on preliminary tests, as the minimum time needed to detect significant changes in solute concentrations and to keep oxygen concentrations at the end of the incubation within 20% of the initial value (Dalsgaard 2000). In particular, the winter and autumn incubations lasted 4 h due to the lower experimental temperatures and lower microbial process rates, while the spring incubation lasted 4 h for the first temperature level (12 °C) and 3 h for the other three experimental temperatures. Finally, summer incubations lasted 2.5 h for the first temperature level (21 °C) and 1.5 h for the other temperatures.
At the end of the incubation period, the O2 concentration in each core was measured in the same manner as that at the beginning. After the first incubation, the water in the tanks was replaced and the cores were submerged for approximately 2 h to stabilize the system. In the second incubation, the isotope pairing technique (IPT; Nielsen 1992) was applied to measure the denitrification and DNRA rates. As in the first incubation, the water level in the tank was lowered to just below the top of the cores to isolate them. An aliquot (0.6 – 1.5 mL according to the water volume in each core and the ambient seasonal NO3− availability) of a stock solution of 15 mM 15NO3− (Na15NO3, Sigma Aldrich, ≥98 atom% enrichment) was added to each core to obtain a final 15NO3− enrichment of 30 – 60%. Then, the cores were capped to start the IPT incubation. The NO3− concentrations were measured in each core before and after the addition of 15NO3− to calculate the 14N:15N ratio in the NO3− pool. The incubation times were the same as those used for the flux incubations and were set to ensure that oxygen consumption was less than 20% of the initial concentration, which is a prerequisite for the IPT method application (Nielsen 1992).
At the end of the incubation period, the entire sediment column was mixed with the water column to homogenize the dissolved N2 pools in the water column and pore water. Slurry samples were transferred to glass-tight vials (12 mL, Exetainer®, Labco Limited, UK), flushing at least 3 times the vial volume, and fixed with 200 μL of 7 M ZnCl2 to stop the microbial activity. The IPT samples were analyzed for 29N2 and 30N2 using membrane inlet mass spectrometry (MIMS) (Bay Instruments, MD, USA; Kana et al. 1994).
An additional aliquot (30 mL) of the slurry from each core was used to determine the DNRA rate from the production of 15 NH4+ (Gervasio et al. 2023; Magri et al. 2022). To determine the exchangeable NH4+ pool, the slurry was treated with 2 g of KCl (2 M), shaken for 30 min, and centrifuged (1800 rpm for 15 min). The supernatant was then filtered through Whatman GF/F glass fiber filters, stored in 20 mL scintillation vials, and frozen for subsequent analysis. Slurry samples were purged with air to eliminate 29N2 and 30N2 pools produced during IPT incubation, transferred to 12 mL-Exetainers (Labco Limited, UK), and treated with an alkaline hypobromite solution to oxidize NH4+ to N2 (Warembourg 1993). After the oxidation procedure, the 29N2 and 30N2 concentrations were measured using MIMS.
Calculation of SOD, denitrification, and DNRA rates
Hourly dark fluxes of O2 (SOD, μmol O2 m−2 h−1) were calculated from the rate of change of concentrations with time according to the following equation (Owens and Cornwell 2016):
where C0 and Cf (μM) are the O2 concentrations at the beginning and end of incubation, respectively, A (m2) is the area of the sediment core, V (L) is the water volume of the sediment core, and t (h) is the incubation time, which was different for each temperature in each seasonal experiment.
Denitrification rates (μmol N m−2 h−1) were calculated on 29N2 and 30N2 production, as the equations below (Nielsen 1992):
where D15 is the denitrification rate of the added 15NO3−, D14 is the total denitrification rate of 14NO3−, and p29 and p30 are the production rates of 29N2 and 30N2, respectively. The total denitrification rate (Dtot) was divided into two components as follows:
where Dw (μmol N m−2 h−1) is the denitrification rate of NO3− diffusing from the water column to the anoxic sediment layer, while Dn (coupled nitrification-denitrification; μmol N m−2 h−1) is the denitrification rate of NO3− produced within the oxic sediment layer by nitrification.
Anammox (anaerobic ammonium oxidation) may interfere with the IPT calculations, leading to an overestimation of the rates, as the N2 produced by anammox cannot be distinguished from that produced by denitrification. Therefore, the independence of the measured genuine 28N2 production from the 15NO3− concentration added to each core was checked to validate the IPT assumptions and to exclude a significant overestimation due to anammox (Risgaard-Petersen et al. 2003). In addition, several studies report that anammox usually accounts for only a small fraction of total N2 production in eutrophic freshwater ecosystems (Koop-Jakobsen and Giblin 2009; Racchetti et al. 2011; Trimmer et al. 2003; Wei and Zhang 2023).
DNRA rates were calculated according to Risgaard-Petersen and Rysgaard (1995), as follows:
where pNH4+ is the production of 15NH4+, DNRAw represents the DNRA of NO3− from the water column, and DNRAn is the DNRA rate coupled to nitrification.
Sediment characterization
The cores for sediment characterization were extracted and sliced into two layers: upper 0–1 cm and 1–2 cm sections. Aliquots from the two layers were rapidly homogenized and subsamples of 5 mL were collected using plastic syringes to determine physical properties (density, volumetric water content, and porosity). Fresh sediments were dried at 50 °C to constant weight for 72 h and then they were set at 350 °C for 3 h into a muffle furnace. The dried samples were used to determine the organic matter content (OM, %) via weight loss during ignition.
Statistical analyses
The correlation between water temperature and benthic fluxes of oxygen was analyzed using a parametric test (Pearson's correlation). The test was performed using the software SigmaPlot 15.0 (Systat Software, Inc., San Jose, CA, USA) and the overall significance level was set at p ≤ 0.05. Benthic fluxes of oxygen, denitrification, and DNRA rates were statistically analyzed using linear mixed effects (LME) to analyze differences in temperature and in seasons. The sample size was equal in all tests. The statistical analyses were run in RStudio (RStudio-2023.06.0-421), using the lme4 package (Douglas Bates et al. 2015). The focus of the test was to compare the differences in seasonal SOD, denitrification, and DNRA rates by taking into account the fixed factors of season (winter, spring, summer, and autumn) and different temperatures in each season, and the correlation between both factors.
Results
Oxygen fluxes
The oxygen concentration measured in situ during the four sampling campaigns ranged from 8.3 mg L−1 in summer, to 11.7 mg L−1 in winter, corresponding to approximately 100% saturation of the water column throughout the year (Table 2). The sediment oxygen demand increased with temperature in all seasonal incubations (Fig. 4). The average values in winter, spring, summer, and autumn were 561 ± 120, 799 ± 159, 1210 ± 116, and 389 ± 120 μmol O2 m−2 h−1, respectively. The raise along the temperature gradient averaged at 60 μmol O2 m−2 h−1 °C−1 in all seasons, except in spring when the highest SOD values were detected. The SOD in spring ranged from 453 ± 42 to 1186 ± 119 μmol O2 m−2 h−1 at 13 and 22 °C, respectively, and increased along the temperature gradient with a raise of 82 μmol O2 m−2 h−1 per degree. In summer incubation, SOD had the highest values due to the highest temperatures, from 936 ± 43 μmol O2 m−2 h−1 at 21 °C to 1937 ± 86 μmol O2 m−2 h−1 at 30 °C. On the contrary, the lowest values appeared during the autumn incubation, when SOD fluxes ranged between 156 ± 39 and 955 ± 84 μmol O2 m−2 h−1 (at 9 and 18 °C, respectively). Finally, the winter SOD ranged from 323 ± 36 to 891 ± 62 μmol O2 m−2 h−1 at 5 and 14 °C, respectively.
The results of the spring experiments were within the range of two extreme seasons, i.e. summer and winter. In fact, the SOD at the highest spring temperature had the same values as those of the summer SOD at the lowest temperature, and the lowest spring temperature had the same values as the highest winter temperature. The lowest values, 156 ± 39 and 216 ± 23 μmol O2 m−2 h−1, were recorded during the autumn period at the first two temperatures tested, 9 and 12 °C, respectively, due to the low experimental temperature and initial O2 concentration in situ (Table 2).
Denitrification and DNRA rates
Denitrification and DNRA rates showed wide seasonal variations related to both NO3− availability and temperature. Total denitrification rates increased along the experimental temperature gradient that was set in each season (Fig. 5). In winter, the total denitrification rates ranged from 38 ± 3 μmol N m−2 h−1 at 5 °C to 186 ± 16 μmol N m−2 h−1 at 14 °C, with a rise of 16 μmol N m−2 h−1 °C−1. The highest rates were measured in spring incubation, increasing from 41 ± 5 to 591 ± 29 μmol N m−2 h−1 at 13 and 22 °C, respectively, with a rise of 61 μmol N m−2 h−1 °C−1. The summer rates increased from 14 ± 8 μmol N m−2 h−1 at 21 °C to 40 ± 5 μmol N m−2 h−1 at 30 °C, with a slight increase, i.e., only 3 μmol N m−2 h−1 °C−1 and, finally, the autumn denitrification rates ranged from 14 ± 5 to 49 ± 14 μmol N m−2 h−1 at 9 and 18 °C, respectively, with a raise of 4 μmol N m−2 h−1 °C−1. Dw dominated Dn, accounting for an average of 76% of Dtot in the winter and spring incubations. In fact, in winter Dw ranged from 38 ± 3 to 127 ± 8 μmol N m−2 h−1 at 5 and 14 °C, respectively, while it showed the highest values in spring incubations, ranging from 30 ± 4 to 482 ± 70 μmol N m−2 h−1 at 13 and 22 °C, respectively. In contrast, in the summer incubations, the Dw (denitrification of NO3− diffusing from the water column to the sediments) was systematically lower than Dn (denitrification coupled to nitrification), representing an average of 31% of Dtot. During the summer experiment, Dn increased along gradient temperature, ranging from 8 ± 3 to 32 ± 3 μmol N m−2 h−1 at 21 and 30 °C, respectively, while the same upward trend was not detected for Dw. Autumn Dw represented 95% of Dtot and increased along the temperature gradient, ranging from 13 ± 5 to 49 ± 15 μmol N m−2 h−1 at 9 and 18 °C, respectively. A strong denitrification response to temperature was observed, with an increase in the temperature gradient in all seasons (p < 0.001; Table 3). Statistical analysis showed a strong correlation between the N process and temperature in each season (Table 3), and the interaction between temperature and season was also significant, ; nevertheless, the average rates differed among seasons (Table 3), due to the different seasonal NO3− availability.
Total denitrification rates (μmol N m−2 h−1) splitted into Dw and Dn measured along the temperature gradients in the four seasons: a winter; b spring, c summer; d autumn (note the different scale of the y-axis). Average values ± standard deviations are reported (n = 5). Dw = denitrification rate of NO3− diffusing from the water column to the anoxic sediment layer. Dn = denitrification rate coupled with nitrification
On average, the denitrification rates were one order of magnitude higher than the DNRA rates. DNRA increased along the temperature gradient in all seasons except winter, when the rates remained constant at the first three temperatures of the series (average 14 μmol N m−2 h−1), increasing up to 31 ± 6 μmol N m−2 h−1 at the last one (14 °C). The highest DNRA rates were measured in spring, ranging from 11 ± 3 to 53 ± 24 μmol N m−2 h−1 at 13 and 22 °C, respectively. The rates in summer and autumn were very low in comparison to the other seasons (Fig. 6; note the difference in scale of the y-axis) and follow a significant linear increase with temperature, from < 2 to 10 ± 1 μmol N m−2 h−1, at 21 and 30 °C, in summer, and from < 2 to 8 ± 1 μmol N m−2 h−1, at 9 and 18 °C, in autumn, respectively.
Total dissimilatory nitrate reduction to ammonium (DNRA) rates (μmol N m−2 h−1) splitted into DNRAw e DNRAn, measured along the temperature gradients in the four seasons: a winter; b spring, c summer; d autumn (note the different scale of the y-axis). Average values ± standard deviations are reported. DNRAw = the direct DNRA of NO3− from the water column. DNRAn = the DNRA rate coupled with nitrification
In winter and spring, DNRAw increased along the temperature gradient (Fig. 6) and represented on average 77 and 64% of DNRAtot, respectively. In summer, the DNRA rates of NO3− diffusing from the water column to the anoxic sediment layer (DNRAw) increased slightly along the temperature gradient but represented less than 30% of DNRAtot. Finally, in autumn, DNRAw increased along the temperature gradient and accounted for >95% of the DNRAtot.
Discussion and conclusion
An increase in the water temperature of a river has several consequences. As water temperature increases, oxygen solubility and diffusion into the sediment decrease (Butcher and Covington 1995; Rajesh and Rehana 2022; Veraart et al. 2011; Muruganandam et al. 2023). Simultaneously, temperature warming stimulates sediment respiration rates and SOD, which in turn results in the vertical extension of the hypoxic-anoxic area within superficial sediments. Thus, this thicker anoxic sediment layer becomes suitable for denitrification because enzymes that sequentially reduce NO3− to N2, i.e., NO3−, NO2−, NO, and N2O reductase (Bonnett et al. 2013; Hobbs et al. 2013; Adouani et al. 2015).
In this study, denitrification responded positively to water warming, increasing along a temperature gradient in each season. There was a significant interaction between temperature and season (Table 3), indicating that the effect of temperature on denitrification was also dependent on other seasonal factors such as NO3− availability (Myrstener et al. 2016), which influenced the partitioning of total denitrification into Dw and Dn. On average, increasing temperature and higher NO3− availability in the water column stimulate anaerobic processes, allowing the contribution of Dw to increase with respect to Dn (Dong et al. 2000). In fact, it has been widely reported that total denitrification rates are mainly supported by Dw when NO3− concentrations in the water column exceed 50 μM (Piña-Ochoa and Álvarez-Cobelas 2006; Nizzoli et al. 2010; Racchetti et al. 2011), according to the winter and spring results. In winter, the total denitrification rates and, consequently, the Dw rates, were low owing to low temperatures, despite the high NO3− concentrations compared with the other seasons. In spring, the total denitrification and Dw rates were the highest due to NO3− availability, increasing along the temperature gradient. In contrast, summer denitrification rates were limited by NO3− availability, despite high temperatures. The exceptionally low flows that characterized the Po River in the summer of 2022 (Montanari et al. 2023) led to a reduction in nutrient runoff from the basin (Cozzi et al. 2018; Viaroli et al. 2018) and consequently limited NO3− availability in water, resulting in a greater relevance of Dn compared to Dw, as previously reported also in the Po delta course, where Dn accounted for >50% of Dtot (Gervasio et al. 2023).
Another exception occurred in autumn, when denitrification rates were low despite the water temperature and NO3− availability (Tables 1, 2). However, although no specific measurements were made in this study, our results support the hypothesis that the sediment content of labile organic matter was lower in autumn (Table 4) than in other seasons, as a result of a decrease in river primary productivity and sedimentation of labile phytoplanktonic material and an increase in lignocellulosic debris transport from the catchment after moderate rainfall, in the week prior to sampling. It is hypothesized that the shift in the ratio of highly biodegradable autochthonous material to refractory lignocellulosic material from the catchment has slowed heterotrophic bacterial metabolism in the sediment and hence NO3− reduction processes (Hu et al. 2019; Warneke et al. 2011). This hypothesis is supported by the low SOD flux measured in autumn (Fig. 4). SOD is strongly related with the availability of labile organic matter in river sediments, the mineralization of which primarily involves oxygen (Hargrave 1972). Thus, oxygen consumption can be considered a proxy for mineralization rates (Seiter et al. 2005; Song et al. 2016). The low SOD in autumn at the same temperatures and with approximately the same sediment total organic matter content measured in spring, when the SOD was almost three times higher, highlights the role of organic matter quality (Myrstener et al. 2016) and variation throughout the year in the Po River.
The denitrification rates measured in this study overlap with those of other large rivers worldwide, although the variability found in the literature studies may be due not only to variable environmental conditions but also to different experimental approaches resulting in rates varying over several orders of magnitude (Piña-Ochoa and Álvarez-Cobelas 2006; Reisinger et al. 2016; Qi and Liu 2023). As recently reviewed by Gervasio et al. (2022), in a global hotspot of NO3− pollution such as the Po River basin, denitrification has been extensively measured in several types of aquatic ecosystems (e.g., wetlands, canals, lagoons, rivers, and lakes) and its regulating factors have been diffusely studied. The range of denitrification rates measured in the sediments of the Po River overlapped with estimates in aquatic environments heavily influenced by the surrounding agricultural landscapes (connected wetlands and rivers), resulting in an increased availability of NO3− and organic carbon, but higher than those found in isolated wetlands, lakes, and coastal lagoons.
Similar to denitrification, DNRA rates were temperature dependent in each seasonal experiment. The total DNRA rates increased along the temperature gradient in each season (Roberts et al. 2014), with the highest rates measured in spring, followed by winter, autumn, and summer DNRA rates. The two contributions of DNRA, i.e. DNRAw and DNRAn, followed the same increase along the temperature gradient, with the exception of DNRAw in summer, due to lower NO3− availability in the water column. In each season, DNRAw dominated DNRAn, except in summer, when the low water NO3- availability due to the extremely dry summer of 2022 resulted in higher DNRAn rates compared to DNRAw. A previous study showed that DNRA exceeded denitrification in organic-rich brackish sediments of the Po River (Gervasio et al. 2023), with rates positively related to the C/N ratio, as a consequence of stimulation by labile carbon availability (Nizzoli et al. 2010). The sediments of the Po River main course, at the section sampled in this study, were poor in organic matter in all seasons (Table 1), particularly the minimum values were measured in summer, highlighting the conditions under which DNRA is not favored (Wei et al. 2020). These results contradict those of other studies showing that increasing temperatures can promote reducing conditions in the sediment by favoring DNRA over denitrification (Yin et al. 2002). In winter, the DNRA had similar values at three of the four experimental temperatures. This was attributed to the high oxygen concentration in the river water column, which is generally well mixed and well-oxygenated, especially in winter (Table 2) (Frascari et al. 2006). High oxygen availability, combined with low sediment organic carbon and low SOD, is such as to result in the complete oxidation of the sandy sediments, limiting the activity of DNRA bacteria to micro-niches and resulting in low rates, that were unaffected by the experimental temperature increase (Kraft et al. 2014). Finally, in autumn, the DNRA rates were low despite NO3− availability (Table 2). This evidence is consistent with the hypothesis previously discussed, regarding the importance of the labile organic matter fraction of the total organic matter in river sediments for DNRA and denitrification (Jiang et al. 2020; Guo et al. 2022; Jaiswal et al. 2023).
Overall, denitrification was found to be the main process responsible for the removal of NO3− from the Po River with a positive trigger from temperature increase, especially in spring when NO3− availability is maximal, as well as the risk of eutrophication in the Adriatic Sea. Instead, the temperature increase did not favor N recycling via DNRA to the extent that it exceeded denitrification, which generally occurs in sediments under strongly reducing conditions (Yuan et al. 2023). In the Po sediments, DNRA contributed on average of 13% to the total NO3− dissimilatory reduction, while most of the NO3− was permanently removed by denitrification, especially in autumn, when denitrification accounted for >90% of the total NO3− removal.
In conclusion, the direct link between water warming induced by climate change and positive denitrification response, described in this study, may have implications for water quality improvement in the Adriatic Sea, due to the potential reduction of N loads especially in spring, when riverine nutrients trigger the most eutrophication. The present outcomes suggest that recent increases in Po River water temperature may have increased the rate of NO3− loss via denitrification in the lowland reach sediments and ultimately caused the downward trends of N loads discharged to the Adriatic Sea, observed in recent decades (Gervasio et al. 2022; Soana et al. 2023). Future studies should seek direct confirmation of this conclusion by extending to the ecosystem scale the experimental rates measured along water temperature gradients in sections characterized by different substrate availability (i.e., NO3− in the water column and labile organic matter in the sediments). Their multiple functional relationships make in fact the effects of climate change on rivers very complex to analyze and difficult to predict. Further research is needed to examine how other climatic factors, such as reduced discharge and extreme rainfall events, may interact with temperature increases in terms of both the dynamics of nutrient load generation and nutrient export to the sea from temperate and Mediterranean basins. This is a key issue for the effective implementation of environmental policies to control eutrophication and protect coastal zones.
References
Aalto SL, Asmala E, Jilbert T, Hietanen S (2021) Autochthonous organic matter promotes DNRA and suppresses N2O production in sediments of the coastal Baltic Sea. Estuar Coast Shelf Sci 255:107369. https://doi.org/10.1016/j.ecss.2021.107369
Abily M, Acuña V, Gernjak W, Rodríguez-Roda I, Poch M, Corominas L (2021) Climate change impact on EU rivers’ dilution capacity and ecological status. Water Res 199:117166. https://doi.org/10.1016/j.watres.2021.117166
Adouani N, Limousy L, Lendormi T, Sire O (2015) N2O and NO emissions during wastewater denitrification step: Influence of temperature on the biological process. Comptes Rendus Chimie 18:15–22. https://doi.org/10.1016/j.crci.2014.11.005
Appiotti F, Krželj M, Russo A, Ferretti M, Bastianini M, Marincioni F (2014) A multidisciplinary study on the effects of climate change in the northern Adriatic Sea and the Marche region (central Italy). Reg Environ Chang 14:2007–2024. https://doi.org/10.1007/s10113-013-0451-5
Ballard TC, Sinha E, Michalak AM (2019) Long-term changes in precipitation and temperature have already impacted nitrogen loading. Environ Sci Technol 53:5080–5090. https://doi.org/10.1021/acs.est.8b06898
Bonaldo D, Bellafiore D, Ferrarin C, Ferretti R, Ricchi A, Sangelantoni L, Vitelletti ML (2023) The summer 2022 drought: a taste of future climate for the Po valley (Italy)? Reg Environ Chang 23(1):1. https://doi.org/10.1007/s10113-022-02004-z
Bonnett SAF, Blackwell MSA, Leah R, Cook V, O’Connor M, Maltby E (2013) Temperature response of denitrification rate and greenhouse gas production in agricultural river marginal wetland soils. Geobiology 11:252–267. https://doi.org/10.1111/gbi.12032
Braga F, Zaggia L, Bellafiore D, Bresciani M, Giardino C, Lorenzetti G, Maicu F, Manzo C, Riminucci F, Ravaioli M, Brando VE (2017) Mapping turbidity patterns in the Po river prodelta using multi-temporal Landsat 8 imagery. Estuar Coast Shelf Sci 198:555–567. https://doi.org/10.1016/j.ecss.2016.11.003
Brin LD, Giblin AE, Rich JJ (2017) Similar temperature responses suggest future climate warming will not alter partitioning between denitrification and anammox in temperate marine sediments. Glob Chang Biol 23:331–340. https://doi.org/10.1111/gcb.13370
Brunetti M, Buffoni L, Mangianti F, Maugeri M, Nanni T (2004) Temperature, precipitation and extreme events during the last century in Italy. Glob Planet Chang 40:141–149. https://doi.org/10.1016/S0921-8181(03)00104-8
Butcher JB, Covington S (1995) Dissolved-oxygen analysis with temperature dependence. J Environ Eng 121:756–759. https://doi.org/10.1061/(ASCE)0733-9372(1995)121:10(756)
Coppola E, Verdecchia M, Giorgi F, Colaiuda V, Tomassetti B, Lombardi A (2014) Changing hydrological conditions in the Po basin under global warming. Sci Total Environ 493:1183–1196. https://doi.org/10.1016/j.scitotenv.2014.03.003
Cozzi S, Ibáñez C, Lazar L, Raimbault P, Giani M (2018) Flow regime and nutrient-loading trends from the largest South European watersheds: implications for the productivity of Mediterranean and Black Sea’s coastal areas. Water 11:1. https://doi.org/10.3390/w11010001
Dalsgaard, T., 2000. Protocol handbook for nitrogen cycling in estuaries: a project under the EU research programme: Marine Science and Technology (MAST III). Ministry of Environment and Energy,. Protocol handbook for nitrogen cycling in estuaries: A project under the EU research programme: Marine Science and Technology (MAST III). ISBN: 9788777725357.
de Klein JJM, Overbeek CC, Juncher Jørgensen C, Veraart AJ (2017) Effect of temperature on oxygen profiles and denitrification rates in freshwater sediments. Wetlands 37:975–983. https://doi.org/10.1007/s13157-017-0933-1
Dodds WK (2006) Eutrophication and trophic state in rivers and streams. Limnol Oceanogr 51:671–680. https://doi.org/10.4319/lo.2006.51.1_part_2.0671
Domeneghetti A, Carisi F, Castellarin A, Brath A (2015) Evolution of flood risk over large areas: quantitative assessment for the Po river. J Hydrol 527:809–823. https://doi.org/10.1016/j.jhydrol.2015.05.043
Dong L, Thornton D, Nedwell D, Underwood G (2000) Denitrification in sediments of the River Colne estuary. England Mar Ecol Prog Ser 203:109–122. https://doi.org/10.3354/meps203109
Douglas Bates MM, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Frascari F, Spagnoli F, Marcaccio M, Giordano P (2006) Anomalous Po River flood event effects on sediments and the water column of the northwestern Adriatic Sea. Clim Res 31(2-3):151–165. https://doi.org/10.3354/cr031151
Gervasio MP, Soana E, Granata T, Colombo D, Castaldelli G (2022) An unexpected negative feedback between climate change and eutrophication: higher temperatures increase denitrification and buffer nitrogen loads in the Po River (Northern Italy). Environ Res Lett 17:084031. https://doi.org/10.1088/1748-9326/ac8497
Gervasio MP, Soana E, Vincenzi F, Magri M, Castaldelli G (2023) Drought-induced salinity intrusion affects nitrogen removal in a deltaic ecosystem (Po River Delta, Northern Italy). Water 15:2405. https://doi.org/10.3390/w15132405
Giambastiani BMS, Colombani N, Greggio N, Antonellini M, Mastrocicco M (2017) Coastal aquifer response to extreme storm events in Emilia-Romagna, Italy. Hydrol Process 31:1613–1621. https://doi.org/10.1002/hyp.11130
Giblin A, Tobias C, Song B, Weston N, Banta G, Rivera-Monroy V (2013) The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanog 26:124–131. https://doi.org/10.5670/oceanog.2013.54
Glibert PM (2017) Eutrophication, harmful algae and biodiversity — challenging paradigms in a world of complex nutrient changes. Mar Pollut Bull 124:591–606. https://doi.org/10.1016/j.marpolbul.2017.04.027
Grilli F, Accoroni S, Acri F, Bernardi Aubry F, Bergami C, Cabrini M, Campanelli A, Giani M, Guicciardi S, Marini M, Neri F, Penna A, Penna P, Pugnetti A, Ravaioli M, Riminucci F, Ricci F, Totti C, Viaroli P, Cozzi S (2020) Seasonal and interannual trends of oceanographic parameters over 40 years in the northern Adriatic Sea in relation to nutrient loadings using the EMODnet chemistry data portal. Water 12:2280. https://doi.org/10.3390/w12082280
Guo Z, Su R, Zeng J, Wang S, Zhang D, Yu Z et al (2022) NosZI microbial community determined the potential of denitrification and nitrous oxide emission in river sediments of Qinghai-Tibetan Plateau. Environ Res 214:114138. https://doi.org/10.1016/j.envres.2022.114138
Hargrave BT (1972) Aerobic decomposition of sediment and detritus as a function of particle surface area and organic content: decomposition of sediment and detritus. Limnol Oceanogr 17:583–586. https://doi.org/10.4319/lo.1972.17.4.0583
Hill AR (2023) Patterns of nitrate retention in agriculturally influenced streams and rivers. Biogeochemistry 163:155–183. https://doi.org/10.1007/s10533-023-01027-w
Hobbs JK, Jiao W, Easter AD, Parker EJ, Schipper LA, Arcus VL (2013) Change in heat capacity for enzyme catalysis determines temperature dependence of enzyme catalyzed rates. ACS Chem Biol 8:2388–2393. https://doi.org/10.1021/cb4005029
Hou C, Chu ML, Guzman JA, Acero Triana JS, Moriasi DN, Steiner JL (2019) Field scale nitrogen load in surface runoff: impacts of management practices and changing climate. J Environ Manag 249:109327. https://doi.org/10.1016/j.jenvman.2019.109327
Hu J, Ouyang W, Yang Z (2023) Impacts of extreme climate on nitrogen loss in different forms and pollution risk with the copula model. J Hydrol 620:129412. https://doi.org/10.1016/j.jhydrol.2023.129412
Hu R, Zheng X, Zheng T, Xin J, Wang H, Sun Q (2019) Effects of carbon availability in a woody carbon source on its nitrate removal behavior in solid-phase denitrification. J Environ Manag 246:832–839. https://doi.org/10.1016/j.jenvman.2019.06.057
Jaiswal D, Naaz N, Gupta S, Madhav K, Pandey J (2023) Diurnal oscillation in dissolved oxygen at sediment-water interface fuels denitrification-driven N removal in Ganga River. J Hydrol 619:129301. https://doi.org/10.1016/j.jhydrol.2023.129301
Jiang X, Gao G, Zhang L, Tang X, Shao K, Hu Y (2020) Denitrification and dissimilatory nitrate reduction to ammonium in freshwater lakes of the Eastern Plain, China: influences of organic carbon and algal bloom. Sci Total Environ 710:136303. https://doi.org/10.1016/j.scitotenv.2019.136303
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal Chem 66:4166–4170. https://doi.org/10.1021/ac00095a009
Koop-Jakobsen K, Giblin AE (2009) Anammox in tidal marsh sediments: the role of salinity, nitrogen loading, and marsh vegetation. Estuar Coasts 32:238–245. https://doi.org/10.1007/s12237-008-9131-y
Kraft B, Tegetmeyer HE, Sharma R, Klotz MG, Ferdelman TG, Hettich RL, Geelhoed JS, Strous M (2014) The environmental controls that govern the end product of bacterial nitrate respiration. Science 345:676–679. https://doi.org/10.1126/science.1254070
Lassaletta L, García-Gómez H, Gimeno BS, Rovira JV (2009) Agriculture-induced increase in nitrate concentrations in stream waters of a large Mediterranean catchment over 25years (1981–2005). Sci Total Environ 407:6034–6043. https://doi.org/10.1016/j.scitotenv.2009.08.002
Magri M, Benelli S, Castaldelli G, Bartoli M (2022) The seasonal response of in situ denitrification and DNRA rates to increasing nitrate availability. Estuar Coast Shelf Sci 271:107856. https://doi.org/10.1016/j.ecss.2022.107856
Marchina C, Natali C, Fazzini M, Fusetti M, Tassinari R, Bianchini G (2017) Extremely dry and warm conditions in northern Italy during the year 2015: effects on the Po river water. Rend Fis Acc Lincei 28:281–290. https://doi.org/10.1007/s12210-017-0596-0
Moatti, J.-P., Thiébault, S. (Eds.), 2016. The Mediterranean region under climate change: a scientific update. IRD Éditions. https://doi.org/10.4000/books.irdeditions.22908
Montanari A (2012) Hydrology of the Po River: looking for changing patterns in river discharge. Hydrol Earth Syst Sci 16:3739–3747. https://doi.org/10.5194/hess-16-3739-2012
Montanari A, Nguyen H, Rubinetti S, Ceola S, Galelli S, Rubino A, Zanchettin D (2023) Why the 2022 Po River drought is the worst in the past two centuries. Sci Adv 9:eadg8304. https://doi.org/10.1126/sciadv.adg8304
Muruganandam M, Rajamanickam S, Sivarethinamohan S, Reddy MK, Velusamy P, Gomathi R et al (2023) Impact of climate change and anthropogenic activities on aquatic ecosystem–a review. Environ Res:117233. https://doi.org/10.1016/j.envres.2023.117233
Myrstener M, Jonsson A, Bergström AK (2016) The effects of temperature and resource availability on denitrification and relative N2O production in boreal lake sediments. J Environ Sci 47:82–90. https://doi.org/10.1016/j.jes.2016.03.003
Nielsen LP (1992) Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol Lett 86:357–362. https://doi.org/10.1111/j.1574-6968.1992.tb04828.x
Nizzoli D, Carraro E, Nigro V, Viaroli P (2010) Effect of organic enrichment and thermal regime on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in hypolimnetic sediments of two lowland lakes. Water Res 44:2715–2724. https://doi.org/10.1016/j.watres.2010.02.002
Oduor BO, Campo-Bescós MÁ, Lana-Renault N, Casalí J (2023) Effects of climate change on streamflow and nitrate pollution in an agricultural Mediterranean watershed in Northern Spain. Agric Water Manag 285:108378. https://doi.org/10.1016/j.agwat.2023.108378
Owens MS, Cornwell JC (2016) The benthic exchange of O2, N2 and dissolved nutrients using small core incubations. JoVE:54098. https://doi.org/10.3791/54098
Penna N, Capellacci S, Ricci F (2004) The influence of the Po River discharge on phytoplankton bloom dynamics along the coastline of Pesaro (Italy) in the Adriatic Sea. Mar Pollut Bull 48:321–326. https://doi.org/10.1016/j.marpolbul.2003.08.007
Piña-Ochoa E, Álvarez-Cobelas M (2006) Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81:111–130. https://doi.org/10.1007/s10533-006-9033-7
Qi H, Liu Y (2023) Nitrogen removal through denitrification in Chinaʼs aquatic system. Sci Total Environ:164317. https://doi.org/10.1016/j.scitotenv.2023.164317
Racchetti E, Bartoli M, Soana E, Longhi D, Christian RR, Pinardi M, Viaroli P (2011) Influence of hydrological connectivity of riverine wetlands on nitrogen removal via denitrification. Biogeochemistry 103:335–354. https://doi.org/10.1007/s10533-010-9477-7
Rajesh M, Rehana S (2022) Impact of climate change on river water temperature and dissolved oxygen: Indian riverine thermal regimes. Sci Rep 12:9222. https://doi.org/10.1038/s41598-022-12996-7
Ravazzani G, Barbero S, Salandin A, Senatore A, Mancini M (2015) An integrated hydrological model for assessing climate change impacts on water resources of the upper Po River basin. Water Resour Manag 29:1193–1215. https://doi.org/10.1007/s11269-014-0868-8
Reisinger AJ, Tank JL, Hoellein TJ, Hall RO Jr (2016) Sediment, water column, and open-channel denitrification in rivers measured using membrane-inlet mass spectrometry. J Geophys Res Biogeosci 121(5):1258–1274. https://doi.org/10.1002/2015JG003261
Risgaard-Petersen N, Nielsen LP, Rysgaard S, Dalsgaard T, Meyer RL (2003) Application of the isotope pairing technique in sediments where anammox and denitrification coexist. Limnol Oceanogr Methods 1(1):63–73. https://doi.org/10.4319/lom.2003.1.63
Risgaard-Petersen N, Rysgaard S (1995) Nitrate reduction in sediments and waterlogged soil measured by 15N techniques. Methods in applied soil microbiology. Academic Press Inc., London, United Kingdom
Roberts KL, Kessler AJ, Grace MR, Cook PLM (2014) Increased rates of dissimilatory nitrate reduction to ammonium (DNRA) under oxic conditions in a periodically hypoxic estuary. Geochim Cosmochim Acta 133:313–324. https://doi.org/10.1016/j.gca.2014.02.042
Romero E, Garnier J, Lassaletta L, Billen G, Le Gendre R, Riou P, Cugier P (2013) Large-scale patterns of river inputs in southwestern Europe: seasonal and interannual variations and potential eutrophication effects at the coastal zone. Biogeochemistry 113:481–505. https://doi.org/10.1007/s10533-012-9778-0
Romero E, Ludwig W, Sadaoui M, Lassaletta L, Bouwman AF, Beusen AHW, Apeldoorn D, Sardans J, Janssens IA, Ciais P, Obersteiner M, Peñuelas J (2021) The Mediterranean region as a paradigm of the global decoupling of N and P between soils and freshwaters. Glob Biogeochem Cycles 35. https://doi.org/10.1029/2020GB006874
Seiter K, Hensen C, Zabel M (2005) Benthic carbon mineralization on a global scale. Glob Biogeochem Cycles 19(1). https://doi.org/10.1029/2004GB002225
Seitzinger SP (1988) Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance: denitrification. Limnol Oceanogr 33:702–724. https://doi.org/10.4319/lo.1988.33.4part2.0702
Silvennoinen H, Liikanen A, Torssonen J, Stange CF, Martikainen PJ (2008) Denitrification and N 2 O effluxes in the Bothnian Bay (northern Baltic Sea) river sediments as affected by temperature under different oxygen concentrations. Biogeochemistry 88:63–72. https://doi.org/10.1007/s10533-008-9194-7
Soana E, Bartoli M, Milardi M, Fano EA, Castaldelli G (2019) An ounce of prevention is worth a pound of cure: managing macrophytes for nitrate mitigation in irrigated agricultural watersheds. Sci Total Environ 647:301–312. https://doi.org/10.1016/j.scitotenv.2018.07.385
Soana E, Gervasio MP, Granata T, Colombo D, Castaldelli G (2023) Climate change impacts on eutrophication in the Po River (Italy): temperature-mediated reduction in nitrogen export but no effect on phosphorus. J Environ Sci:S100107422300308X. https://doi.org/10.1016/j.jes.2023.07.008
Song G, Liu S, Zhu Z, Zhai W, Zhu C, Zhang J (2016) Sediment oxygen consumption and benthic organic carbon mineralization on the continental shelves of the East China Sea and the Yellow Sea. Deep-Sea Res II Top Stud Oceanogr 124:53–63. https://doi.org/10.1016/j.dsr2.2015.04.012
Speir SL, Tank JL, Taylor JM, Grose AL (2023) Temperature and carbon availability interact to enhance nitrous oxide production via denitrification in alluvial plain river sediments. Biogeochemistry 165:191–203. https://doi.org/10.1007/s10533-023-01074-3
Spillman CM, Imberger J, Hamilton DP, Hipsey MR, Romero JR (2007) Modelling the effects of Po River discharge, internal nutrient cycling and hydrodynamics on biogeochemistry of the Northern Adriatic Sea. J Mar Syst 68:167–200. https://doi.org/10.1016/j.jmarsys.2006.11.006
Struglia MV, Mariotti A, Filograsso A (2004) River discharge into the Mediterranean Sea: climatology and aspects of the observed variability. J Clim 17:4740–4751
Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P, van Grinsven H, Grizzetti B (eds) (2011) The European nitrogen assessment: sources, effects and policy perspectives, 1st ed. Cambridge University Press. https://doi.org/10.1017/CBO9780511976988
Trimmer M, Nicholls JC, Deflandre B (2003) Anaerobic ammonium oxidation measured in sediments along the Thames estuary, United Kingdom. Appl Environ Microbiol 69(11):6447–6454. https://doi.org/10.1128/AEM.69.11.6447-6454.2003
Triska FJ, Higler LB (2009) Biogeochemical processes in river systems. Fresh Surface Water 2:175
Tu J (2009) Combined impact of climate and land use changes on streamflow and water quality in eastern Massachusetts, USA. J Hydrol 379:268–283. https://doi.org/10.1016/j.jhydrol.2009.10.009
Velthuis M, Veraart AJ (2022) Temperature sensitivity of freshwater denitrification and N2O emission—a meta-analysis. Glob Biogeochem Cycles 36. https://doi.org/10.1029/2022GB007339
Veraart AJ, De Klein JJM, Scheffer M (2011) Warming can boost denitrification disproportionately due to altered oxygen dynamics. PLoS One 6:e18508. https://doi.org/10.1371/journal.pone.0018508
Vezzoli R, Mercogliano P, Pecora S, Zollo AL, Cacciamani C (2015) Hydrological simulation of Po River (North Italy) discharge under climate change scenarios using the RCM COSMO-CLM. Sci Total Environ 521–522:346–358. https://doi.org/10.1016/j.scitotenv.2015.03.096
Viaroli P, Soana E, Pecora S, Laini A, Naldi M, Fano EA, Nizzoli D (2018) Space and time variations of watershed N and P budgets and their relationships with reactive N and P loadings in a heavily impacted river basin (Po river, Northern Italy). Sci Total Environ 639:1574–1587. https://doi.org/10.1016/j.scitotenv.2018.05.233
Warembourg FR (1993) Nitrogen fixation in soil and plant systems. Academic Press Inc. ed. R Knowles and T H Black-burn, New York, NY, USA
Warneke S, Schipper LA, Matiasek MG, Scow KM, Cameron S, Bruesewitz DA, McDonald IR (2011) Nitrate removal, communities of denitrifiers and adverse effects in different carbon substrates for use in denitrification beds. Water Res 45:5463–5475. https://doi.org/10.1016/j.watres.2011.08.007
Wei C, Zhang W (2023) Nitrogen contribution rate of anammox in different systems and its relationship with environmental factors. Water 15(11):2101. https://doi.org/10.3390/w15112101
Wei H, Gao D, Liu Y, Lin X (2020) Sediment nitrate reduction processes in response to environmental gradients along an urban river-estuary-sea continuum. Sci Total Environ 718:137185. https://doi.org/10.1016/j.scitotenv.2020.137185
Yin SX, Chen D, Chen LM, Edis R (2002) Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biol Biochem 34:1131–1137. https://doi.org/10.1016/S0038-0717(02)00049-4
Yuan H, Cai Y, Wang H, Liu E, Zeng Q (2023) Impact of seasonal change on dissimilatory nitrate reduction to ammonium (DNRA) triggering the retention of nitrogen in lake. J Environ Manag 341:118050. https://doi.org/10.1016/j.jenvman.2023.118050
Zanchettin D, Traverso P, Tomasino M (2008) Po River discharges: a preliminary analysis of a 200-year time series. Clim Chang 89:411–433. https://doi.org/10.1007/s10584-008-9395-z
Zhang Q, Huang J, Zhang J, Qian R, Cui Z, Gao J (2024) Characterizing nitrogen dynamics and their response to sediment dredging in a lowland rural river. J Hydrol 628:130479. https://doi.org/10.1016/j.jhydrol.2023.130479
Zheng J, Cao X, Ma C, Weng N, Huo S (2023) What drives the change of nitrogen and phosphorus loads in the Yellow River Basin during 2006-2017? J Environ Sci 126:17–28. https://doi.org/10.1016/j.jes.2022.04.039
Acknowledgements
The authors thank Prof. Marco Bartoli and Dr. Monia Magri (University of Parma, Italy) for their assistance with the DNRA measurements.
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement. This study was financially supported by the Consorzio di Bonifica Pianura di Ferrara (Ferrara Plain Reclamation Consortium) as part of a long-term collaboration aimed at defining management strategies to control eutrophication in the Po Delta region. This research was also made possible thanks to funding from the Emilia-Romagna Region (Hunting and Fisheries Division) in the framework of the project “Assessment and mapping of the productivity of the bivalve mollusc aquaculture in the Sacca di Goro lagoon and the coastal stretch from Lido di Volano to Lido delle Nazioni, Ferrara, Emilia-Romagna.”
Author information
Authors and Affiliations
Contributions
Maria Pia Gervasio: investigation, formal analysis, writing original draft preparation, visualization; Elisa Soana: methodology, conceptualization, writing review and editing, supervision; Anna Gavioli: formal analysis; Fabio Vincenzi: investigation; Giuseppe Castaldelli: conceptualization, writing review and editing, funding acquisition, supervision
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gervasio, M.P., Soana, E., Gavioli, A. et al. Contrasting effects of climate change on denitrification and nitrogen load reduction in the Po River (Northern Italy). Environ Sci Pollut Res 31, 48189–48204 (2024). https://doi.org/10.1007/s11356-024-34171-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34171-3