Abstract
Solvent-based CO2 capture is a commonly employed post-combustion technique in processes involving absorber-stripper columns. This study focused on computer simulations with equilibrium- and rate-based modeling of CO2 capture using the amine solvents 2-amino-2-methyl-1-propanol (AMP), diethanolamine (DEA), and methyl diethanolamine (MDEA) and thermodynamic methods involving electrolyte NRTL models. The objective of this study was to understand the impacts of rate-based modeling, the type of amine, and thermodynamic methods on carbon capture. Within this study, the amine-based CO2 capture process from coal-power plant flue gas was studied using Aspen Plus modeling. Simulations were also conducted to determine the impact of thermodynamics and kinetics on the CO2 capture performance of the system. The results were analyzed on the basis of captured CO2 according to the solvents and models. The equilibrium approach was mostly invalid because of the oversimplified ideal stage assumptions through the column. The lowest carbon capture capacity was obtained with MDEA, while DEA yielded the best results. A sensitivity analysis with rate-based modeling showed the significant impact of the inlet CO2 composition. The amine-based CO2 capture process simulation included solution chemistry, electrolyte thermodynamics, rigorous transport property modeling, reaction kinetics, and rate-based multistage simulation, which could be applicable to different solvent systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental concerns and decarbonization
Anthropogenic factors are the fundamental cause behind global climate disruption and the emission of greenhouse gases (GHGs), which are responsible for disastrous consequences. These consequences include global warming, a decrease in Arctic sea and land ice, and a relative sea level rise, which have been observed during recent decades, endangering life on Earth. Consequently, various environmental directives have been implemented in an effort to eliminate adverse climatic factors and related consequences (Bompard et al. 2018; Rockström et al. 2017; Kopac 2023; Succar et al. 2023).
Efforts are increasing worldwide to significantly decrease GHG emissions as of 2050 in compliance with the Paris Agreement on Climate Change, which requires transformational measures (Sundaramoorthy et al. 2023; UNFCCC 2023). Specifically, regarding global warming, the importance of substantial GHG emission mitigation for decades to come has been highlighted, namely, to reach or approach near-zero levels by the close of the century, ensuring a temperature increase less than 2 °C relative to pre-industrial levels, particularly in an effort to constrain it to 1.5 °C by 2050 (Bompard et al. 2018; Hou et al. 2023; IPCC 2023; Rockström et al. 2017; Steffen et al. 2015; Succar et al. 2023). From 2017, the global mean temperature rise amounted to almost 1 °C and is estimated to reach the 1.5-°C indication as of 2040 in the case of these trends. A temperature limit of 2 °C implies attaining the zero emissions target in the latter half of this decade; however, when restricting the temperature to 1.5 °C, GHG emissions need to converge a nearly zero level as of 2050 (Rissman et al. 2020; Sundaramoorthy et al. 2023).
The decarbonization strategy encompasses various approaches, such as expanding the use of RESs; implementing carbon capture, utilization, and storage (CCUS) systems; promoting almost energy-efficient buildings; developing smart grids; implementing carbon pricing (taxing carbon emissions per ton of CO2); fostering CO2-free processes; and utilizing CO2 from external sources for the production of goods. These strategies play a pivotal role in reducing carbon emissions. The variety of RESs, including biomass, solar, wind, geothermal, and other emerging zero/low-carbon energy sources, is expected to expand (Bompard et al. 2018; Kopac 2021a; Chen et al. 2023; Nesterenko et al. 2023; Succar et al. 2023; Zheng and Jin 2023).
Carbon capture technologies
It has been reported that nearly 79% of all CO2 emissions result from the fossil fuels combustion and the use of minerals for power production and that coal-powered plants play an important role in global warming (Aghaie et al. 2018; IPCC 2023; Kopac 2021b; Yu et al. 2008). CO2 emissions rely upon the content of the fuel (Madejski et al. 2022). Among the main constituents of flue gas resulting from fuel combustion, such as unburned fuel, CO2, N2O, N2, and SO2 currently exist largely in the atmosphere at continually rising levels exceeding 400 ppm (Varghese et al. 2020; Varghese and Karanikolos 2020). Hence, eliminating CO2 from flue gases is considered important for reducing carbon levels in the atmosphere. The utilization of fossil fuels, including coal, oil, and natural gas, obviously keeps going, so it would be appropriate to use them following the application of effective strategies for carbon mitigation (Aghaie et al. 2018). Many countries still use coal-powered systems due to their lower cost and safety, which cannot be completely substituted with RES operating systems (Madejski et al. 2022).
Carbon capture methods can be categorized into three primary groups: pre-combustion process, oxy-combustion, and post-combustion (Aghaie et al. 2018; Madejski et al. 2022; Arshad and Alhajaj 2023; Baudoux et al. 2024).
Pre-combustion methods are applied prior to combustion by means of gasification of fuel with oxygen, whereas oxy-combustion carbon capture methods are employed after combustion in the presence of oxygen by separating CO2 formed during oxy-combustion treatment. Post-combustion is used after combustion to capture CO2 from flue gas. This can be accomplished through diverse approaches, such as membrane separation, physical adsorption, chemical absorption, or chemical looping (Madejski et al. 2022). The main distinction between post-combustion and oxy-combustion methods lies in the composition of the flue gas. In oxy-combustion, the flue gas is intensely concentrated in CO2, making it appropriate for underground storage. Alternatively, the post-combustion technique requires additional procedures for carbon capture from flue gas (Aghaie et al. 2018; Chen et al. 2012). As the utilization of fossil fuels in current technologies continues until alternative fuels are replaced without CO2 emissions, CO2 capture is inevitable for reducing GHGs (Madejski et al. 2022).
The post-combustion technique for the capture of CO2 is more appropriate as an alternative for retrofitting in power plants than are the oxy-combustion and pre-combustion capture strategies, which can be implemented only in newly established power plants (Aghaie et al. 2018; Chen et al. 2012; Sreedhar et al. 2017a). However, the main barrier in the application of these methods is that since the CO2 partial pressure in flue gas is low, the driving force for CO2 would also be low (Wang et al. 2017). Post-combustion carbon capture methods depend on eliminating CO2 from flue gas, with the capturing unit installed following the purification system, such as desulfurization, denitrogenation, or dust removal units (Madejski et al. 2022).
Post-combustion technologies can be subdivided into several categories, such as absorption, microbial (algal), membrane or physical separation, adsorption, chemical looping for combustion, and cryogenic methods. Among those methods, absorption-based CO2 capture is among the commonly employed methods in commercial carbon capture plants due to its lower energy requirements and efficiency (Aghaie et al. 2018; Madejski et al. 2022; Mostafavi et al. 2021; Sreedhar et al. 2017a). Through this technique, the flue gas is treated by contacting it with a solvent in the absorption towers, which enables CO2 capture from the flue gas. The CO2 absorption rate of a solvent is an essential factor in the chemical absorption process. Higher absorption rates can result in capital cost savings for the process, as well as influencing the operation of the process at an industrial level (Aghaie et al. 2018; Sreedhar et al. 2017b).
Amine-based CO2 capture
The physical absorption technique depends on the use of chemically inert solvents, such as organic solvents (methanol, N-methyl-2-pyrrolidone, or dimethyl ether), or simply water absorbing CO2 physically. Optimal results can be accomplished by operating at reduced temperatures and increased pressures for the separated gas (Madejski et al. 2022).
Chemical absorption is a well-known technique for CO2 capture based on reactions between CO2 and chemical solvents. This process has long been employed in chemical industries; however, it is energy intensive. Chemical absorption methods are utilized in solid fuel-fired power plants as the only selections available commercially. According to previous reports, amine methods possess the capability to capture approximately 85–95% of CO2 from flue gas at levels exceeding 99.95% purity (Madejski et al. 2022).
Different types of solvents for chemical absorption–based carbon capture have been used (Aghaie et al. 2018; Rochelle 2009). Among those, amine-based CO2 capture has found widespread industrial applications, including H2S removal, syngas purification, and various other processes, including those applied in the steel industry for decreasing large-scale CO2 emissions (Luo et al. 2016; Varghese and Karanikolos 2020; Ma’mun et al. 2007; Nielsen et al. 2012; Zhou et al. 2011). The amine-based carbon capture approach utilizes an amine solvent for the absorption of CO2 from flue gas. Subsequently, the CO2 is desorbed from the solvent, allowing for its reuse after regeneration (Zhou et al. 2011).
The solvents that are typically employed as absorbents for carbon capture operations include alkanolamines, including monoethanolamine (MEA) and diethanolamine (DEA); tertiary amines, such as MDEA; and sterically hindered amines, for example, AMP in aqueous solutions (Madejski et al. 2022), because of their strong reactivity toward CO2 molecules, significant absorption capacity, and temperature durability. Conversely, the process of amine-based carbon capture involves rather complicated work involving the follow-up of a number of operating variables, flow rate, pressure, temperature, and level of reaction instruments, as well as careful manipulation of numerous valves and pumps, directly impacting plant performance and CO2 capture efficiency (Zhou et al. 2011). Additionally, the application of amines has certain drawbacks, including equipment corrosion, high construction costs, and amine degradation by O2, NO2, and SO2 in flue gas. Consequently, absorbent regeneration processes at high temperatures require a significant amount of absorbent makeup and result in increased energy consumption. These drawbacks are correlated with the intrinsic features of amines, such as their corrosive nature, elevated vapor pressures, and substantial energy requirements for regeneration (Aghaie et al. 2018; Torralba-Calleja et al. 2013). Furthermore, conventional frameworks often suffer drawbacks, such as limited capacity to increase amine concentration, a highly diffusion-constrained process, and environmental pollution resulting from amine discharge (Nielsen et al. 2012).
Piperazine (PZ), MEA, and MDEA are among the widely employed amine solvents in industrial processes (Mostafavi et al. 2021). Moreover, lower energy expenditure was reported for PZ-AMP solvents and ammonia, which deserves further investigation (Madejski et al. 2022). Furthermore, MEA exhibits satisfactory absorption and desorption even when blended with different kinds of solvents (Chao et al. 2021). In addition to conventional solvents (MEA, DEA, PZ, ammonia), other solvents have been devised for CO2 capture processes. Blends of various solvents can potentially enhance absorptive properties through the use of proper combinations of various types of adsorbents. Primary and secondary amines indicate elevated absorption rates, while tertiary amines are identified by greater absorption (Madejski et al. 2022; Nord and Bolland 2020).
Simulation studies on absorption-based CO2 capture
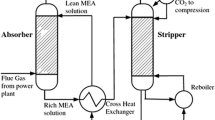
Reports show that the most appropriate solutions for existing coal-fired units are post-combustion methods (Madejski et al. 2022). Numerous investigations have been carried out to simulate the amine-based CO2 capture process. In general, the simulations involved a pre-treatment stage, comprising cooling down the flue gas by eliminating impurities involving particulate matter and other gaseous impurities, such as NOx and SOx. After pre-treatment, the flue gas is injected into the absorber, where it is exposed to the lean solvent. Next, the solvent, rich in CO2, is introduced into the stripping column, where CO2 extraction from the solvent and regeneration of the lean solvent take place. The lean solvent is subsequently sent back to the absorbing column for further utilization in the procedure. Finally, the high-purity CO2 stream from the desorber can then be exposed to drying and post-treatment. It can be compressed and stored or subsequently employed (Madejski et al. 2022; Zhou et al. 2011).
Aspen Plus modeling of a post-combustion CO2 capture process for a coal-fueled power plant with PZ-enhanced K2CO3 solution (K2CO3/PZ) was studied by Oexmann et al. (2008). A sensitivity analysis was performed on the important operational parameters, including lean loading, solvent composition, CO2 capture rate, and desorption pressure, to identify optimal parameters that would minimize overall specific energy requirements. A stagewise equilibrium model was employed for modeling the absorber and desorber units, and the outcomes from these calculations were utilized to design the column sizing for each theoretical stage while accounting for kinetic effects. Analysis revealed significant energy savings by employing K2CO3/PZ with compression for the capture process in comparison to the conventional MEA process and lower investment costs because of the improved reaction kinetics, resulting in smaller component sizes.
Pellegrini et al. (2010) conducted an Aspen Plus modeling study to assess the CO2 capture efficiency of various solvents (MEA, DGA, and NH3) in a flue gas stream. The simulation used a conventional absorption–desorption unit configuration with RadFrac-type columns for chemical equilibrium calculations. The results indicated that ammonia was an effective absorbent, achieving effective removal efficiencies with minimal solvent use but required energy to reduce ammonia emissions.
Arachchige and Melaaen (2012) focused on simulating CO2 removal from flue gas streams for gas and coal fuel power plants using MEA solvent in Aspen Plus. With an 85% CO2 removal rate, the rate-based model utilized the electrolyte non-random two-liquid (ELECNRTL) method for simulating the reactive capture process and optimizing its settings. The reboiler duty decreased with increasing absorption column pressure and packing height, while the removal efficiency increased with increasing temperature and solvent concentration. Nevertheless, the efficiency of the removal process decreased with increasing lean loading of MEA solvent.
Jana and De (2014) proposed an integrated gasification combined cogeneration system utilizing sugarcane bagasse with CO2 capture using an aqueous MEA solution. This study utilized Aspen Plus for thermodynamic modeling, enabling a comparison between the pre- and post-combustion CO2 capture approaches. For the physical property determination of the conventional components involved in the system, the Peng–Robinson equation of state with an alpha function was utilized. This equation yields accurate results for correlating the vapor pressure of pure components at high temperatures due to the temperature-dependent variable, alpha. When estimating the thermo-physical properties of the process, the ELECNRTL property method demonstrated consistent reliability. The results indicated that the post-combustion capture process was the best pathway. Furthermore, the capture process had to be optimized based on the overall plant performance, as an increased capture level affects both the thermodynamic efficiency and cost-effectiveness of the system.
Lungkadee et al. (2021) carried out simulation studies for retrofitting a PCCC unit with a power plant (300 MW) using MEA as the solvent and reported the CO2 capture costs (≤ 55 $/ton). The absorber and stripping units were designed to have a 90% CO2 capture capacity with MEA (30 wt%). Approximately 63.075 kg/s of CO2 capture from flue gas (458 kg/s) was reported (Lungkadee et al. 2021). A simulation study of a natural gas combined cycle power plant utilizing PZ as a solvent demonstrated superior performance in comparison to MEA. Using PZ (40 wt%) exhibited noteworthy enhancements in capture effectiveness, energy utilization, and costs in comparison to employing MEA (30 wt%). The CO2 capture costs (min 34.65 $/ton) of the analysis with PZ solvent (40 wt%) were reported (Otitoju et al. 2021). El Hadri et al. (2017) studied various amine solutions (30 wt%) for PCCCs. Hexamethylenediamine showed the optimal CO2 loading, while triethanolamine showed the minimum CO2 loading.
Chuenphan et al. (2021) studied Aspen Plus modeling of CO2 capture using MEA with equilibrium-based methods and the ENRTL-RK thermodynamic property model. An experimental design involving a 2k factorial methodology was implemented to examine the influence of several parameters, including the sour gas temperature, liquid-to-gas mass ratio, sour gas CO2 concentration, and lean MEA concentration and temperature, on CO2 capture and the reboiler heat duty. The study reported the optimization of these parameters for improving the process performance.
Sultan et al. (2021) evaluated the techno-economic analysis of the CO2 capture process for coal fuel power plants using an MEA solution. The conventional process and different stripper modifications (stripper overhead exchanger, lean vapor compression, and a hybrid configuration) were studied using the Aspen Plus rigorous rate-based model for simulating the absorber and stripper and the Aspen Process Economic Analyzer for optimizing the process. The ENRTL property model and Redlich–Kwong (RK) equation of state were applied for modeling the liquid (L) phase and vapor (V) phase, respectively. All of these modifications resulted in reduced energy consumption and demonstrated economic benefits. Using the optimal hybrid configuration (LVCSOE), both the solvent regeneration energy consumption and CO2 capture costs were reduced. An economic analysis revealed that, compared with other economic parameters, CO2 capture costs were most affected by regeneration steam costs.
Aim of the present study
In our research, we focused on examining the significance of various thermodynamic models, as well as equilibrium- and rate-based models, to represent the reaction kinetics and the impact of different solvents in capturing CO2. While most studies in this field rely on established equilibrium modeling, our study delved into the importance of incorporating kinetic modeling and the specific role that different solvents play in the CO2 capture process. The novelty of the study lies in its comprehensive comparison of different amine solvents, evaluation of thermodynamic models, emphasis on rate-based modeling, and the analysis of CO2 inlet composition in capturing CO2. These aspects contribute to a deeper understanding of carbon capture processes and provide valuable insights for optimizing efficiency and for a further economic feasibility analysis.
The simulation studies were carried out for chemical absorption–based CO2 capture systems with different aqueous amine solvents, namely, AMP, DEA, and MDEA. In this framework, the performances of solvents, AMP, DEA, and MDEA were studied based on a flowsheet involving an absorber and stripper units. The impact of thermodynamics and kinetics on chemical absorption–based CO2 capture with respect to different amine solvents was investigated. For a steady evaluation of the efficacy of various amine solvents, the physical properties, kinetics, and thermodynamics need to be verified according to the requirements of Aspen Plus modeling. For the systems, parametric simulations were conducted to determine the impact of thermodynamics and kinetics on the carbon capture performance of the amine-based CO2 capture system.
Materials and methods
The study utilizes a comprehensive methodology consisting of computer simulations, modeling, and data analysis to explore the decarbonization of flue gas through solvent-based CO2 capture. To achieve this, the following steps in the methodology were taken: a thorough literature review to establish the foundation and identify research gaps, utilization of Aspen Plus simulation software to model the carbon capture process, comparison of different amine solvents (AMP, DEA, and MDEA), use of thermodynamic methods (ELECNRTL, ENRTL-RK, ENRTL-HF, and ENRTL-HG), use of equilibrium- and rate-based models to evaluate solvent behavior and CO2 capture performance, analysis of mass flows to evaluate the performance of different solvents, calculation of CO2 capture percentage and sensitivity analysis to determine the impact of CO2 mole fraction in the feed gas on carbon capture performance, data analysis to compare results, identify trends, and draw conclusions. The methodology aims to provide insights into optimizing the carbon capture process by combining theoretical modeling, computer simulations, and data analysis.
Modeling and simulation studies
Thermodynamic property models
In the simulations, the thermodynamic property methods and the reaction kinetic models utilized were obtained from the following sources: Aspentech (2014a) and Jamal et al. (2006a, b) for AMP; Aspentech (2014b) and Rinker et al. (1996) for DEA; and Aspentech (2020a), Austgen et al. (1991), Rinker et al. (1997), and Pinsent et al. (1956) for MDEA.
The thermodynamic models that were utilized in the simulations to depict the CO2 capture system with Aspen Plus modeling included true species, including ions, and electrolyte transport property models, which are described as follows.
ELECNRTL model: The electrolyte NRTL model, coupled with the RK equation of state, has been used for applications that involve aqueous and mixed solvents. This property model in Aspen Plus, which was first proposed by Chen et al. (1979) and Chen et al. (1982) and subsequently extended by Chen and Evans (1986) and Mock et al. (1986), can be used for determining the concentrations of aqueous and solvent mixtures. The model can be used for modeling the VLE of electrolyte systems and evaluating the excess Gibbs free energy of an electrolyte solution. The excess Gibbs free energy of the electrolyte system was modeled by combining short-range (ion-ion, molecule–molecule, local ion–molecule) and long-range (Pitzer–Debye–Huckel/Born) interactions (Chen et al. 1982; Chen and Evans 1986; Austgen 1989; Pitzer 1980; Robinson and Stokes 1970). The local interaction contributions are obtained in accordance with the NRTL model. The sole tunable model parameters involve the empirical binary energy interaction parameters and need to be evaluated via regression of available system data (Austgen 1989, Kothandaraman 2010).
ENRTL-RK model: This model combines the non-symmetric electrolyte NRTL model with the RK equation and Henry’s law which is suitable for electrolyte systems with a symmetric reference state for all components.
ENRTL-HF model: This model couples the electrolyte NRTL model with the HF equation of state which is ideal for mixed solvent applications.
ENRTL-HG model: This model utilizes the electrolyte NRTL model with the RK equation, incorporating the Helgeson model to estimate standard properties and equilibrium constants.
The equilibrium models as well as concentration-based reaction kinetics and rate-based models for the absorption and stripping columns involving packing were used in the simulations.
System overview
In the simulations for the CO2 capture processes by the three types of amine solvents (AMP/DEA/MDEA), the operational data of a pilot plant reported originally by Gabrielsen (2007; Aspentech 2014a) were utilized for the system composed of the absorber and stripper units. The typical sets of operation data employed in the simulations are presented in Table 1.
Physical properties
The abovementioned property models (ELECNRTL, ENRTL-RK, ENRTL-HF, and ENRTL-HG) and the equation of states were employed for computations of V and L properties in equilibrium- and rate-based models with the solvents AMP/DEA/MDEA.
Henry’s law was utilized for a selection of Henry components (solutes), including N2, O2, CO, H2, H2S, and CO2. Henry’s parameters for the components were determined using water and the solvents AMP/MDEA.
In the computations for the reaction, the basis selection for activity coefficients regarding Henry’s components was as aqueous. Hence, in determining the asymmetric activity coefficients of solutes, infinite dilution activity coefficients can be computed under conditions of unlimited dilution in pure water rather than in blended solvents.
Henry’s parameters were obtained from the Aspen Plus database for the components associated with water (Aspentech 2014a, b; Aspentech 2020a). Henry’s parameters for CO2 in water were estimated by regression from binary VLE data (Takenouchi and Kennedy 1964; Tödheide and Franck 1963; Dodds et al. 1956; Drummond 1981; Zawisza and Malesiska 1981; Wiebe and Gaddy 1940; Houghton et al. 1957). Henry’s constants of CO2 in AMP/MDEA solvents were acquired from the study conducted by Wang et al. (1992) (Aspentech 2014a; Aspentech 2020a). Henry’s constants in DEA were calculated by regression from CO2 solubility data (Maddox et al. 1987; Maddox and Elizondo 1989), H2S solubility data (Barreau et al. 2006), and literature (Lawson and Garst 1976) for H2S (Aspentech 2014b).
The NRTL interaction parameters for H2O-CO2 were assigned as zero, while those of H2O-AMP were calculated from regressions with binary VLE (Pappa et al. 2006), excess enthalpy (Mathonat et al. 1997), and heat capacity data (Chen and Li 2001; Chiu and Li 1999; Zhang et al. 2002). The NRTL interaction parameters for H2O-MDEA were determined via regression with binary VLE (Xu et al. 1991; Voutsas et al. 2004; Kim et al. 2008), excess enthalpy (Posey 1996; Maham et al. 1997, 2000), and heat capacity data (Chiu and Li 1999; Chen et al. 2001; Zhang et al. 2002). The NRTL parameters were calculated by regression of CO2 solubility (Maddox et al. 1987; Maddox and Elizondo 1989) and H2S solubility data for DEA (Barreau et al. 2006; Lawson and Garst 1976).
The interaction energy parameters for H2O-(AMPH+, HCO3−) were regressed from the CO2 heat of solution data in aqueous AMP (Arcis et al. 2007) and CO2 solubility data in aqueous AMP solution. The data (Robert and Mather 1988; Tontiwachwuthikul et al. 1991; Teng and Mather 1990; Li and Chang 1994; Seo and Hong 1992; Jane and Li 1997; Kundu et al. 2003) were used in the regression for CO2 solubility in AMP. The VLE data with high CO2 loading (loading > 1) were not included in the regression, so this model was applicable to VLE from low to moderate CO2 loading. The interaction energy parameters for H2O-(AMPH+, HS−) were obtained by regression utilizing H2S solubility data in aqueous AMP solution (Roberts and Mather 1988).
The interaction parameters for H2O-(MDEAH+, HCO3−), H2O-(MDEAH+, CO3−2), and MDEA-(MDEAH+, HCO3−) were calculated by regression from the ternary VLE data (Kuranov et al. 1996; Kamps et al. 2001; Ermatchkov et al. 2006; Jou et al. 1982, 1993a), CO2 absorption heat data (Mathonat 1995; Carson et al. 2000), ternary heat capacity data (Weiland et al. 1997), and MDEA-H2O-CO2 liquid-phase concentration data (Bottinger et al. 2008). The energy parameters for H2O-(MDEAH+, HS−) and MDEA-(MDEAH+, HS−) were estimated via regression from H2S solubility data in aqueous MDEA solution (Kuranov et al. 1996; Kamps et al. 2001; Huang and Ng 1998).
The calculation results of the transport and thermal properties and model parameters utilized in the simulations, such as the liquid viscosity, density, and thermal conductivity; surface tension (AMP-CO2-H2O; DEA-CO2-H2O; MDEA-CO2-H2O) (Weiland et al. 1998; Weiland 1996); liquid heat capacity (AMP-H2O; DEA-CO2-H2O; MDEA-CO2-H2O) (Chen and Li 2001; Weiland et al. 1997); excess enthalpy (AMP-H2O) (Mathonat et al. 1997); integral heat of solution (AMP-CO2-H2O; DEA-CO2-H2O; MDEA-CO2-H2O); VLE (AMP-H2O; MDEA-CO2-H2O) of the investigated systems; CO2 partial pressure (AMP-CO2-H2O; DEA-CO2-H2O); and H2S partial pressure (AMP-H2S-H2O; DEA-H2S-H2O), which were adapted according to the experimental data from the literature are summarized in Table 2.
Reactions
AMP is a hindered primary amine where the amino group is linked to a tertiary carbon atom. DEA is a secondary amine with two ethanol groups connected to the nitrogen atom, and MDEA is a tertiary amine with a methyl group and two ethanol groups bonded to the nitrogen atom (Wang et al. 2004). AMP can react with H3O+ to form AMPH+. It may also associate with CO2 to form unstable carbamate, which readily reacts with other species in solution, ending with AMPH+. DEA can also react with H3O+ to form DEAH+ ions. It may also undergo a reaction with CO2 to form the carbamate ion DEACOO−. MDEA can react with H+ to form MDEAH+ ions. However, it is incapable of reacting with CO2 to generate carbamates, such as primary or secondary ethanolamines. The molecular, structural, and 3D images of AMP, DEA, and MDEA are shown in Table 3.
The RadFrac distillation model in Aspen Plus12 offers both equilibrium- and rate-based modeling options that can be easily switched between two modes depending on the user selection used in the simulations. The equilibrium-stage approach for modeling distillation problems is among the most commonly used models in process simulators, as the solution algorithms are well-established and robust for a wide range of systems. Because of the ubiquity of equilibrium-based models, it is convenient to specify efficiency factors to introduce non-equilibrium behavior into equilibrium-stage models. For equilibrium-stage models, vaporization or Murphree efficiencies for stages or for column sections and component efficiencies for specific components in a given stage or column section can be specified. Although convenient, the use of equilibrium-stage models with vaporization and Murphree efficiencies has certain limitations, such as the variation in efficiency factors with column conditions (flow, temperature, pressure) and poor efficiency in reactive systems, as mass transfer due to kinetic or equilibrium reactions is not related to the phase equilibrium driving force and is also due to the requirement of numerous adjustable factors (Aspentech 2020b).
For the equilibrium-stage modeling approach in this present investigation, the electrolyte solution chemistries were simulated with CHEMISTRY Model identifiers, called CHEMISTRY ID = AMP, CHEMISTRY ID = DEA, and CHEMISTRY ID = MDEA. The CHEMISTRY IDs shown in Table 4 were used in the simulations. All the ionic reactions involved in the CHEMISTRY models identified by AMP, DEA, and MDEA given in Table 3 were assumed to be in chemical equilibrium.
As an alternative to using vaporization and Murphree efficiencies with the equilibrium modeling approach, RadFrac also provides a rigorous rate-based modeling option that avoids some of the shortcomings linked to the efficiency approach (Aspentech 2020b). The rate-based model is particularly favorable for packed columns; absorption and desorption processes; reactive separation techniques involving reactive absorption and distillation, and strongly non-ideal systems; columns with complex configurations (such as those with pumparounds and side streams); and columns with both trays and packing (Aspentech 2017).
The rate-based feature of RadFrac in Aspen Plus enables rate-based simulation of absorption and stripping columns (Aspentech 2014a, b; Aspentech 2020a, b). The rate-based distillation approach utilizes heat and mass transfer correlations, adapted from the tray/packing geometry and the transfer properties, to analyze the column behavior. This eliminates the need for efficiency factors (Aspentech 2020b). It is based on various stages allowing the modeling of the kinetics of chemical reactions along with the heat and mass transfer phenomena involved. The several equations that can be solved include heat and mass balance equations for the L and V phases, heat and mass transfer rate models for calculating interphase transfer rates, VL equilibrium expressions for the interphase, calculations of heat and mass transfer coefficients and interfacial areas, and improvements in heat and mass transfer operations by chemical reactions (Aspentech 2008). The model solves the multicomponent Maxwell–Stefan mass transfer equation using two-film theory, in combination with the film and separate balance equations for the VL phases, reaction kinetics and diffusion, thermodynamics, and electrolyte solution chemistry. The hydrodynamics of the column are considered by using correlations for mass transfer coefficients, interfacial area, holdup, and pressure drop (Zhang et al. 2009; Kothandarama 2010; Aspentech 2017; Aspentech 2020b).
For the rate-based modeling approach in the present study, REACTION models called AMP-REA, DEA-REA, and MDEA-REA were established, as shown in Table 5; these models are used in the reaction calculations of the absorption and stripping columns. In the AMP-REA, DEA-REA, and MDEA-REA models, all of the reactions are considered to be in a state of chemical equilibrium except for the CO2-OH− and CO2-AMP reactions and the CO2-DEA and CO2-MDEA reactions.
AMP
Equilibrium constants for reactions 1–5 in the AMP model were determined from the changes in the standard Gibbs free energy. The values for the aqueous phase free energy, heat of formation, and heat capacity at infinite dilution of AMPH+ and AMPCOO− were utilized to calculate the standard Gibbs free energies of these components. The values for the other components, including the aqueous phase free energy, heat of formation, and heat capacity at infinite dilution, were extracted from the databank of Aspen Plus. Equilibrium constants for reactions 6–7 in the AMP model were sourced from the literature (Austgen et al. 1989).
The power law expression given by Eq. (1) was utilized for the rate-based reactions (reactions 4-7 of the AMP-REA model). In general, the mathematical expression for the power law can be given as:
In Eq. (1), r is rate of reaction; k shows the pre-exponential factor; T corresponds to the temperature (absolute); T0 is the reference temperature; E, activation energy; n, exponent for temperature; R, gas constant; Ci, concentration; N, number of components in the reaction; and ai, stoichiometric coefficient of components. When T0 is unspecific, the general power law expression given by Eq. (1) is reduced to the Eq. (2):
In the present simulations, the reduced power law expression provided by Eq. (2) is utilized. The molarity basis of the concentration was used in this equation, and the exponent n = 0, pre-exponential factor k, and activation energy E are provided in Table 6. The kinetic constants for reaction 4 were extracted from the literature (Pinsent et al. 1956), while those of reaction 5 were computed according to the values of the kinetic constants of reaction 4 and the equilibrium constants of the equilibrium reactions 4 and 5. The second-order reaction kinetics expression for reaction 6 is used for the reaction between AMP and CO2, which is a simplification of the expression given by Jamal et al. (2006a, b). The kinetic parameters for reaction 6 given in Table 6 are those of k1 given by Jamal et al. (2006a, b), corresponding to the forward reaction rate constant for zwitterion formation.
The kinetic parameters of reaction 7 were determined from the values of the kinetic parameters of reaction 6 and the equilibrium constants of reactions 6 and 7.
DEA
The equilibrium expressions for the reactions are obtained from the literature (Austgen et al. 1989; Jou et al. 1982; Jou et al. 1993a, b). The reduced form of the power law expression (Eq. 2) is employed for the kinetic reactions (reactions 4-7 of the DEA-REA model). The concentration was on a molarity basis, and the exponent n was taken as zero. The k and E values are tabulated in Table 6. The kinetic parameters for reactions 4-7 given in Table 5 are based on Rinker et al. (1996), provided that the ranges of DEA concentration (0.25–2.8 M) and temperature (292–343 K) correspond to the process conditions of the simulation system of the present study. The kinetic parameters for reaction 4 were sourced from the literature (Pinsent et al. 1956), while those of reaction 5 were computed utilizing the kinetic parameters of reaction 4 and the equilibrium constants of equilibrium reactions 4 and 5.
MDEA
Equilibrium constants for reactions 1–4 of the MDEA model were computed using the standard Gibbs free energy changes. The values of the aqueous phase free energy and heat of formation and the heat capacity at infinite dilution of MDEAH+ were determined and utilized to calculate the standard Gibbs free energy of MDEAH+. For the other components, the values of the parameters aqueous phase free energy and heat of formation and the heat capacity at infinite dilution were obtained from the software databank. The equilibrium constants for reactions 5–6 (MDEA model) were taken from the literature (Austgen et al. 1989). The reduced form of the power law equation was utilized for kinetic reactions 4, 5, 8, and 9 of the MDEA-REA model (Eq. 2). In the equation, the exponent n is zero, and the values of k and E are presented in Table 6. The kinetic parameters for reaction 4 given in Table 5 are obtained from the literature (Pinsent et al. 1956), while those of reaction 5 are computed by utilizing the kinetic parameters of reaction 4 and the equilibrium constants of the equilibrium reactions 4 and 5. The kinetic parameters for reaction 8 were extracted from Rinker et al. (1997), and those of reaction 9 were computed utilizing the kinetic parameters of reaction 8 and the equilibrium constants of equilibrium reactions 8 and 9.
Simulation approach
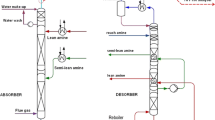
Simulation studies were conducted utilizing experimental data from a pilot plant for CO2 absorption with AMP solution with structured packing in both absorption and stripping columns, as reported by Gabrielsen (2007). The modeling was carried out according to the simulation flowsheet in Aspen Plus, as indicated in Fig. 1. The major unit operations, as shown by the Aspen Plus blocks, and the specifications used in the modeling are described in Table 7. The correlations accounted for the heat (Chilton and Colburn 1934) and mass transfer coefficients, interfacial area (Bravo et al. 1985), and holdup (Bravo et al. 1992) utilized in modeling are also listed in the table.
The feed specifications and feed stream conditions for the absorption and stripping columns are supplied in Table 8. GASIN is the feed entering the absorber and contains N2, CO2, and H2O. LEANIN is the liquid lean solvent stream to the absorber containing aqueous AMP/DEA/MDEA solution. The feed to the stripper is a rich solvent stream, RICHIN, that contains aqueous AMP/DEA/MDEA solution with absorbed CO2.
Results and discussion
Effect of solvent type, thermodynamics models, and rate versus equilibrium models on carbon capture
Traditionally, absorption and desorption columns can be modeled through equilibrium models in which the columns are segmented into several stages under the assumption that the L and V phases departing from a stage in a column are in a state of equilibrium (Treybal 1981). Nevertheless, this oversimplified assumption is mostly invalid in real instances. Therefore, corrections need to be made by employing parameters such as stage and Murphee efficiencies and the height equivalent to a theoretical plate (Taylor et al. 2003). However, specifically for reactive separation operations, the utilization of efficiency factors does not occur correctly as the discrepancies from the equilibrium models become too large (Aspiron 2006). Therefore, under such circumstances, the implementation of rate-based models for modeling systems is required (Kothandarama 2010). The rate-based modeling approach offers several advantages in comparison to the equilibrium-stage modeling. These models offer a more precise representation of the system and yield more realistic simulation results than equilibrium-stage models. This improved accuracy reduces the risk of inadequate designs or deviations from desired operating conditions. Rate-based models explicitly consider the specific column configuration, which directly influences the column efficiency. Although the rate-based model is more complex as compared to the equilibrium model, its complexity is hidden in the background. However, configuring a RateSep simulation is easy for users. RateSep includes several embedded correlations for holdup and mass transfer, supports film reactions and film discretization, and can accurately solve reactive separation problems. The application of the model can be extended to model novel situations with greater accuracy (Aspentech 2017).
The rate-based approach considers that separation occurs due to mass transfer between the interacting phases. The Maxwell–Stefan theory is utilized for calculating the rates of mass transfer. Conversely, the equilibrium model takes into account the equilibrium of the contacting phases. However, this assumption is critically flawed as the contacting phases could never be in equilibrium in real columns (Aspentech 2017).
As per the literature, the rate-based model is generally considered to produce more reliable results than the equilibrium model when applied to empirical data in reactive separation systems. Therefore, for reliable system simulations, the rate-based model ought to be the preferred option for reactive separation processes (Eckert and Vanek 2001; Springer et al. 2002; Taylor et al. 2003; Aspentech 2017). For instance, Kenig et al. (2001) reported that the rate-based modeling approach accurately predicts concentration profiles in H2S scrubbers, whereas the equilibrium model may yield inconsistent results compared to those of experimental studies. Eckert and Vanek (2001) reported that the rate-based model outperforms the equilibrium model in predicting concentration profiles, particularly in the middle of three-phase distillation columns. In the modeling of sour gas absorption by aqueous amine solutions, the rate-based model precisely predicts both composition and temperature profiles (Kucka et al. 2003). Klöker et al. (2005) employed rate-based and equilibrium models for reactive separation processes and discovered that the rate-based model, with fine discretization, better matches the column bottom concentration and temperature profile. The rate-based modeling approach has proven successful in modeling numerous industrial separation processes, including reactive distillation (Klöker et al. 2005; Dhale et al. 2004), reactive absorption (Kucka et al. 2003; Klöker et al. 2005; Bolhàr-Nordenkampf et al. 2004), reactive stripping (Klöker et al. 2005), and three-phase distillation (Repke et al. 2004; Higler et al. 2004).
In this study, the simulations were conducted using Aspen Plus12. The critical simulation findings are outlined in Tables 9, 10 and 11 for the solvents AMP, DEA, and MDEA, respectively. A comparison of the results for the ELECNRTL, ENRTL-RK, ENRTL-HF, and ENRTL-HG thermodynamic models for both the rate and equilibrium models is presented for each of the solvents in the tables. For the absorber and stripper units, the total mass flow rates and the CO2 mass flow rates in kilograms per hour corresponding to GASIN and GASOUT are shown.
For the assessment of CO2 emission levels and to measure the ability of CO2 capture to control emissions, a number of indicators, depending on the choice, can be used (Manzolini et al. 2020; Voldsund et al. 2019). The most widely used ones are the specific emission of CO2, relative emissivity of CO2, CO2 capture ratio, CO2 emission index, CO2 captured, CO2 avoided, CO2 emitted, specific primary energy consumption cost for prevented CO2, and levelized costs of electricity (Madejski et al. 2022; Nord and Bolland 2020).
In this study, CO2 captured was used as an indicator for the assessment of CO2 emissions. The CO2 captured percentage (%) in the absorber unit was calculated as follows:
Table 9 presents simulation results for the AMP solvent. When the ELECNRTL thermodynamic model was used with the rate-based model, CO2 capture was found to be 90.82%, while it was 96.66% for the equilibrium model, indicating a discrepancy of 6.43% between the results of the models, evaluated as the absolute value of the difference between the CO2 capture from the equilibrium model and the CO2 capture from the rate-based model, divided by the CO2 capture from the rate-based model. Similarly, for the rate-based model, when the ENRTL-RK thermodynamic model was used, the CO2 capture rate was determined to be 95.51%, whereas it was 95.43% for the equilibrium model, indicating a discrepancy of only 0.08%. For the rate-based model with the ENRTL-HF thermodynamic model, CO2 capture was 91.56%, whereas it was 96.66% for the equilibrium model, indicating a discrepancy of 5.57%. Finally, for the rate-based model with the ENRTL-HG thermodynamic model, CO2 capture was 92.34%, whereas it was 97.21% for the equilibrium model, indicating a discrepancy of 5.27%. Table 10 summarizes the results for DEA. When the ELECNRTL thermodynamic model was used with the rate-based model, CO2 capture was found to be 98.23%, while for the equilibrium model, it was 92.11%, indicating a discrepancy of 6.23%. Similarly, for the rate-based model, when the ENRTL-RK thermodynamic model was used, the CO2 capture rate was determined to be 99.95%, whereas it was 99.97% for the equilibrium model, indicating a discrepancy of just 0.68%. For the rate-based model with the ENRTL-HF thermodynamic model, CO2 capture was 98.29%, whereas it was 98.39% for the equilibrium model, indicating a discrepancy of just 0.09%. Finally, for the rate-based model with the ENRTL-HG thermodynamic model, CO2 capture was 98.46%, whereas it was 98.56% for the equilibrium model, indicating a discrepancy of just 0.1%. Table 11 presents the results for MDEA. CO2 capture was found to be 67.88% for the rate-based model with ELECNRTL thermodynamic model, whereas it was 81.24% for the equilibrium model. This indicates a discrepancy of 19.68%. Similarly, for the rate-based model with the ENRTL-RK thermodynamic model, CO2 capture was found to be 76.97%, whereas it was 83.63% for the equilibrium model, indicating a discrepancy of 8.65%. For the rate-based model with the ENRTL-HF thermodynamic model, CO2 capture was found to be 71.97%, whereas it was 81.40% for the equilibrium model, indicating a discrepancy of 13.10%. Finally, for the rate-based model with the ENRTL-HG thermodynamic model, CO2 capture was 77.22%, whereas it was 83.70% for the equilibrium model, indicating a discrepancy of 8.39%.
The impact of the type of solvent used on carbon capture has been analyzed and the results for all the models corresponding to all the solvents are presented in Table 12. The carbon capture results obtained with AMP for different thermodynamic, rate, and equilibrium models are presented in Fig. 2. Figure 3 shows the results for carbon capture with DEA solvent for the different thermodynamic models and rate and equilibrium models, and Fig. 4 shows the results for the solvent MDEA. It is evident that for the solvent AMP, higher CO2 capture results in the absorber were obtained for the equilibrium models when the ELECNRTL, ENRTL-HF, and the ENRTL-HG thermodynamic models were used, while ENRTL-RK gave similar CO2 capture results for both the rate and the equilibrium models. For the MDEA solvent, higher CO2 capture results in the absorber were obtained for the equilibrium models when the ELECNRTL, ENRTL-RK, ENRTL-HF, and ENRTL-HG thermodynamic models were used. It is worth noting that traditional models assume that the absorption and desorption columns are in a state of equilibrium, in which the columns are segmented into several stages assuming that the L and V phases departing from one stage in the column are in a state of equilibrium. However, this oversimplified assumption is mostly inaccurate in real instances; specifically, for reactive separation operations, the discrepancies from the equilibrium models become too large. Therefore, applying rate-based models for modeling the systems is needed under such circumstances.
For solvent DEA, higher CO2 capture results in the absorber were acquired for the rate-based models when the ELECNRTL thermodynamic model was applied, while the ENRTL-HF, the ENRTL-HG, and the ENRTL-RK gave similar CO2 capture values for both the equilibrium and rate models. It has been reported in the literature that secondary amines exhibit less corrosiveness and demand less heat for regeneration. This is attributed to the additional ethanol group, which, when compared to primary amines, diverts a significant portion of the free electron character away from the nitrogen atom (Wang et al. 2004; Gunasekaran et al. 2013).
It is evident that the lowest carbon capture values for the absorber unit were obtained with the solvent MDEA. Higher carbon capture values were obtained with the AMP solvent than with MDEA. The optimal outcomes were achieved with the solvent DEA for all the thermodynamic models applied, with similar results for both the rate and the equilibrium models.
According to the simulation results, the solvents can be ranked in terms of their carbon capture performance, with DEA being the most effective, followed by AMP and MDEA. The lowest carbon capture values were obtained with the MDEA solvent. In comparison, higher carbon capture values were obtained with the AMP solvent than MDEA. However, the optimal outcomes were achieved with the DEA solvent for all thermodynamic property models applied. Both the rate and equilibrium models produced similar results. In fact, the best results were obtained with the DEA solvent when using the rate-based model with the ENRTL-RK thermodynamic property model. This produced a carbon capture value of 99.95%, with a very low discrepancy of only 0.68% when compared to the equilibrium model. For the AMP solvent, the rate-based model with the ENRTL-RK thermodynamic model produced a CO2 capture rate of 95.51%, compared to 95.43% with the equilibrium model, indicating a discrepancy of only 0.08%. DEA has several advantages, such as low vapor pressure, minimal reactivity with COS and CS2, and low corrosiveness compared to primary amines. However, it does have some drawbacks, including difficulty reclaiming contaminants, inadequate treatment of gas streams with high CO2 levels, and the formation of corrosive degradation products when combined with CO2 (Luo et al. 2016). On the other hand, AMP, a hindered amine, has decent performance and some advantages, such as ease of regeneration compared to MEA, high CO2 loading, excellent CO2 absorption, higher degradation resistance, and low corrosion rates. However, it also has some disadvantages, such as lower CO2-amine mass transfer rates than MEA and larger substituents that cause its carbamate to be unstable, making it easy to form a bicarbonate. The MDEA solvent had the lowest CO2 capture performance based on simulation results. However, it does have some advantages, such as selective capture of H2S in the presence of CO2, good performance in concentrations up to 60% in aqueous solutions, resistance to degradation, low corrosiveness, low specific heat and heat of reaction with H2S and CO2, and thin miscibility with hydrocarbons. Additionally, it has a better distribution of driving force due to the nature of the gas–liquid equilibria, and it does not react with COS and CS2, resulting in low solvent loss. However, it does have a disadvantage in that it has a lower heat of reaction and a slow reaction with CO2. According to a prior experimental study by Luo et al. (2016), a fast screening method was tested on amine-based solvents for post-combustion CO2 capture. The study examined the effectiveness of single amines, including MEA, MDEA, AMP, and DEA, in aqueous solutions. The findings indicated that AMP and DEA outperformed MDEA in terms of CO2 capture. These results were further confirmed by Aspen Plus process simulations conducted under identical experimental conditions for each of the amines (Luo et al. 2016). Despite the outstanding CO2 capture performance of certain amines, selecting the appropriate solvent system remains a costly and time-consuming process, considering all aspects.
Sensitivity analysis for determining the effect of the GASIN CO2 composition
Based on previous calculations, the CO2 mole fraction in GASIN was selected to be 0.129. In order to assess the influence of the CO2 mole fraction on CO2 capture, the calculations were repeated for different values of the CO2 mole fraction in GASIN, specifically 0.22, 0.32, and 0.42, using both the solvent DEA and the ELECNRTL rate-based model. Table 13 displays the impact of the CO2 inlet composition (GASIN) on the ELECNRTL rate-based model for the solvent DEA. Additionally, Table 14 provides a comparative analysis of the effect of the CO2 inlet composition (GASIN) on the final carbon capture in the absorber unit for the ELECNRTL rate-based model using the DEA solvent. Furthermore, Fig. 5 visually represents the influence of the CO2 feed gas composition on carbon capture with DEA for the ELECNRTL and rate-based models. The figure highlights the favorable impact of the CO2 composition in GASIN, demonstrating that higher carbon capture results can be achieved as the CO2 mole fraction in GASIN increases.
Conclusions and future perspectives
In this study, the process of CO2 capture from a coal-fired power plant flue gas involving absorption and stripping units has been investigated using Aspen Plus equilibrium- and rate-based models, as well as various thermodynamic models, to examine the effectiveness of the aqueous amine solvents AMP, DEA, and MDEA in capturing CO2. The study utilized a comprehensive methodology consisting of computer simulations, modeling, and data analysis to explore the decarbonization of flue gas through solvent-based CO2 capture.
The simulation results show that DEA is the most effective solvent for carbon capture, followed by AMP and MDEA. The lowest carbon capture values were obtained with the MDEA solvent. The best results were achieved with the DEA solvent for all thermodynamic property models applied. The rate-based model with the ENRTL-RK thermodynamic property model produced the optimal outcomes for both DEA (99.95%) and AMP solvents (95.51%), with very low discrepancies in comparison to the equilibrium model. The study’s findings show a positive effect of the inlet CO2 composition, indicating that higher levels of carbon capture can be attained as the concentration of CO2 in the inlet increases.
The rate-based AMP-DEA-MDEA models provided a rate-based, rigorous simulation of the chemical absorption–based CO2 capture process. The notable features of the simulation included solution chemistry and electrolyte thermodynamics, rigorous transport property modeling, liquid-phase reaction kinetics, and rate-based multistage simulation with Aspen rate-based distillation incorporating heat and mass transfer correlations, taking into consideration the details of the absorbing and stripping columns and hydraulic characteristics. The methodology provided insights into optimizing the carbon capture process by combining theoretical modeling, computer simulations, and data analysis.
The model can be applied for modeling the CO2 capture process with different solvents other than AMP, DEA, and MDEA solvents and various blends of solvents. These studies can be extended to evaluate the cost of CO2 capture systems using diverse solvent systems.
These processes can be analyzed further, specifically by examining how essential operational variables such as solvent loading and composition, desorption pressure, and the CO2 capture rate affect the energy cost and the overall cost of capturing CO2 (expressed in cost/ton CO2 captured). In a techno-economic analysis, both capital costs (investment, installation, construction, and engineering) and operating costs (such as solvent makeup, maintenance, insurance, and taxes) could be considered. All process configurations could be compared to the conventional process regarding energy requirements, capital expenditures, and capture costs. Beyond the economic analysis, sensitivity analysis could also be conducted to gain a deeper comprehension of how economic variables impact the cost of CO2 capture.
References
Aghaie M, Rezaei N, Zendehboudi S (2018) A systematic review on CO2 capture with ionic liquids: current status and future prospects. Renew Sustain Energy Rev 96:502–525. https://doi.org/10.1016/j.rser.2018.07.004
Arachchige USPR, Melaaen MC (2012) Aspen Plus simulation of CO2 removal from coal and gas fired power plants. Energy Procedia 23:391–399. https://doi.org/10.1016/j.egypro.2012.06.060
Arcis H, Rodier L, Coxam JY (2007) Enthalpy of solution of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol. J Chem Thermodynamics 39:878–887. https://doi.org/10.1016/j.jct.2006.11.011
Arshad N, Alhajaj A (2023) Process synthesis for amine-based CO2 capture from combined cycle gas turbine power plant. Energy 274:127391. https://doi.org/10.1016/j.energy.2023.127391
Aspentech (2014a) Aspen Plus, rate-based model of the CO2 capture process by AMP using Aspen Plus. Aspen Technology Inc, Bedford, MA
Aspentech (2014b) Aspen Plus, rate-based model of the CO2 capture process by DEA using Aspen Plus. Aspen Technology Inc, Bedford, MA
Aspentech (2020a) Aspen Plus, rate-based model of the CO2 capture process by MDEA using Aspen Plus. Aspen Technology Inc, Bedford, MA
Aspentech (2020b) Aspen Plus, Can you compare using a rate-based distillation model vs. using an equilibrium stage model with efficiencies? Article ID: 000085297. Aspen Technology Inc, Bedford, MA
Aspentech, (2008) Aspen Plus. User Online Documentation, Aspen Technology Inc, Cambridge, MA
Aspentech, (2017) Aspen Plus, Why is rate-based distillation better than using equilibrium stages with efficiencies? Article ID: 000084842. Aspen Technology Inc, Bedford, MA
Aspiron N (2006) Nonequilbirum rate-based simulation of reactive systems: simulation model, heat transfer, and influence of film discretization. Ind Eng Chem Res 45:2054–2069. https://doi.org/10.1021/ie050608m
Augsten DM (1989) A model for vapor-liquid equilibria for acid gas-alkanolamine-H2 O systems. University of Texas at Austin, Dissetation
Austgen DM, Rochelle GT, Chen CC (1991) Model of vapor-liquid equilibria for aqueous acid gas-alkanolamine systems. 2. Representation of hydrogen sulfide and carbon dioxide solubility in aqueous MDEA and carbon dioxide solubility in aqueous mixtures of MDEA with MEA and DEA. Ind Eng Chem Res 30:543–555. https://doi.org/10.1021/ie00051a016
Austgen DM, Rochelle GT, Peng X, ChenCC, (1989) Model of vapor-liquid equilibria for aqueous acid gas-alkanolamine systems using the electrolyte-NRTL equation. Ind Eng Chem Res 28:1060–1073. https://doi.org/10.1021/ie00091a028
Barreau A, Blanchon le Bouhelec E, Habchi Tounsi KN, Mougin P, Lecomte F (2006) Absorption of H2S and CO2 in alkanolamine aqueous solution: experimental data and modelling with the electrolyte-NRTL model. Oil Gas Sci Technol – Rev. IFP 61:345–361. https://doi.org/10.2516/ogst:2006038a
Baudoux A, Demeyer F, Paepe WD (2024) Advanced configurations of amine based post-combustion carbon capture process applied to combined cycle gas turbine. Energy Conv Manag: X 22:100537. https://doi.org/10.1016/j.ecmx.2024.100537
Bolhàr-Nordenkampf M, Friedl A, Koss U, Tork T (2004) Modeling selective H2S absorption and desorption in an aqueous MDEA-solution using a rate-based non-equilibrium approach. Chem Eng Process 43:701–715. https://doi.org/10.1016/S0255-2701(03)00011-4
Bompard E, Grosso D, Huang T, Profumo F, Lei X, Li D (2018) World decarbonization through global electricity interconnections. Energies 11:1746. https://doi.org/10.3390/en11071746
Bottinger W, Maiwald M, Hasse H (2008) Online NMR spectroscopic study of species distribution in MDEA-H2O-CO2 and MDEA-PIP-H2O-CO2. Ind Eng Chem Res 47:7917–7926. https://doi.org/10.1021/ie800914m
Bravo JL, Rocha J, Fair JR (1985) Mass transfer in gauze packings. Hydrocarb Process 64:91–95
Bravo JL, Rocha JA, Fair JR (1992) A comprehensive model for the performance of columns containing structured packings. Inst Chem Eng Symp Ser 128:A489–A507
Carson JK, Marsh KN, Mather AE (2000) Enthalpy of solution of carbon dioxide in (water + monoethanolamine, or diethanolamine, or N-methyldiethanolamine) and (water + monoethanolamine + N-methyldiethanolamine) at T = 298.15 K. J Chem Thermodyn 32:1285–1296. https://doi.org/10.1006/jcht.2000.0680
Chao C, Deng Y, Dewil R, Baeyens J, Fan X (2021) Post-combustion carbon capture. Renew Sustain Energy Rev 138:110490. https://doi.org/10.1016/j.rser.2020.110490
Chen CC, Evans LB (1986) A local composition model for the excess Gibbs energy of aqueous electrolyte systems. AIChE J 32:444–454. https://doi.org/10.1002/aic.690320311
Chen YJ, Li MH (2001) Heat capacity of aqueous mixtures of monoethanolamine with 2-amino-2-methy-1-propanol. J Chem Eng Data 46:102–106. https://doi.org/10.1021/je000146d
Chen CC, Britt HI, Boston JF, Evans LB (1979) Extension and application of the Pitzer equation for vapor-liquid equilibrium of aqueous electrolyte systems with molecular solutes. AIChE J 25:820–831. https://doi.org/10.1002/aic.690250510
Chen CC, Britt HI, Boston JF, Evans LB (1982) Local composition model for excess Gibbs energy of electrolyte systems. Part I: single solvent, single completely dissociated electrolyte systems. AIChE J 28:588–596. https://doi.org/10.1002/aic.690280410
Chen YJ, Shih TW, Li MH (2001) Heat capacity of aqueous mixtures of monoethanolamine with N-methyldiethanolamine. J Chem Eng Data 46:51–55. https://doi.org/10.1021/je0000367
Chen Y, Mutelet F, Jaubert JN (2012) Modeling the solubility of carbon dioxide in imidazolium-based ionic liquids with the PC-SAFT equation of state. J Phys Chem B 116:14375–14388. https://doi.org/10.1021/jp309944t
Chen J, Jia J, Wang L, Zhong C, Wu B (2023) Carbon reduction countermeasure from a system perspective for the electricity sector of Yangtze River Delta (China) by an extended logarithmic mean Divisia index (LMDI). Systems 11:117. https://doi.org/10.3390/systems11030117
Chilton TH, Colburn AP (1934) Mass transfer (absorption) coefficients prediction from data on heat transfer and fluid friction. Ind Eng Chem 26(11):1183–1187. https://doi.org/10.1021/ie50299a012
Chiu LF, Li MH (1999) Heat capacity of alkanolamine aqueous solutions. J Chem Eng Data 44:1396–1401. https://doi.org/10.1021/je990131j
Chuenphan T, Yurata T, Sema T, Chalermsinsuwan B (2021) Sensitivity analysis by the 2k factorial experimental design of CO2 capture with amine gas treating process using Aspen Plus. Eng J 25:95–104. https://doi.org/10.4186/ej.2021.25.4.95
Dhale AD, Myrant LK, Chopade SP, Jackson JE, Miller DJ (2004) Propylene glycol and ethylene glycol recovery from aqueous solution via reactive distillation. Chem Eng Sci 59:2881–2890. https://doi.org/10.1016/j.ces.2004.02.018
Dodds WS, Stutzman LF, Sollami BJ (1956) Carbon dioxide solubility in water. Ind Eng Chem 1:92–95. https://doi.org/10.1021/i460001a018
Drummond SE (1981) Boiling and mixing of hydrothermal fluids: chemical effects on mineral precipitation. PhD Thesis, Pennsylvania State University, University Park, Pa
Eckert E, Vanek T (2001) Some aspects of rate-based modeling and simulation of three-phase distillation columns. Comput Chem Eng 25:603–612. https://doi.org/10.1016/S0098-1354(01)00640-8
El Hadri N, Quang DV, Goetheer ELV, Abu Zahra MRM (2017) Aqueous amine solution characterization for post-combustion CO2 capture process. Appl Energy 185:1433–1449. https://doi.org/10.1016/j.apenergy.2016.03.043
Ermatchkov V, Kamps APS, Maurer G (2006) Solubility of carbon dioxide in aqueous of N-methyldiethanolamine in the low gas loading region. Ind Eng Chem Res 45:6081–6091. https://doi.org/10.1021/ie0604270
Gabrielsen J (2007) CO2 capture from coal fired power plants. PhD Thesis, Technical University of Denmark, Lyngby, Denmark
Gunasekaran P, Veawab A, Aroonwilas A (2013) Corrosivity of single and blended amines in CO2 capture process. Energy Procedia 37:2094–2099. https://doi.org/10.1016/j.egypro.2013.06.088
Higler A, Chande R, Taylor R, Baur R, Krishna R (2004) Nonequilibrium modeling of three-phase distillation. Comput Chem Eng 28:2021–2036. https://doi.org/10.1016/j.compchemeng.2004.04.008
Hou J, Yue Y, Wang Y, Ye J (2023) Decarbonization without borders: evidence from China. Sci Total Environ 892:164653. https://doi.org/10.1016/j.scitotenv.2023.164653
Houghton G, Mclean AM, Ritchie PD (1957) Compressibility, fugacity, and water-solubility of carbon dioxide in the region 0–36 atm and 0–100 C. Chem Eng Sci 6:132–137. https://doi.org/10.1016/0009-2509(57)85006-4
Huang SH, Ng HJ (1998) Solubility of H2S and CO2 in alkanolamines. GPA Research Report No. 155, September 1998
IPCC (2023) AR5 Climate Change 2014: mitigation of climate change—IPCC. Available online: https://www.ipcc.ch/report/ar5/wg3/. Accessed on 5 June 2023
Jamal A, Meisen A, Lim CJ (2006a) Kinetics of carbon dioxide absorption and desorption in aqueous alkanolamine solutions using a novel hemispherical contactor – I. Experimental apparatus and mathematical modeling. Chem Eng Sci 61:6571–6589. https://doi.org/10.1016/j.ces.2006.04.046
Jamal A, Meisen A, Lim CJ (2006b) Kinetics of carbon dioxide absorption and desorption in aqueous alkanolamine solutions using a novel hemispherical contactor – II. Experimental results and parameter estimation. Chem Eng Sci 61:6590–6603. https://doi.org/10.1016/j.ces.2006.04.047
Jana K, De S (2014) Biomass integrated gasification combined cogeneration with or without CO2 capture – a comparative thermodynamic study. Renew Energy 72:243–252. https://doi.org/10.1016/j.renene.2014.07.027
Jane IS, Li MH (1997) Solubilities of mixtures of carbon dioxide and hydrogen sulfide in water + diethanolamine + 2-amino-2-methyl-1-propanol. J Chem Eng Data 42:98–105. https://doi.org/10.1021/je960270q
Jou FY, Mather AE, Otto FD (1982) Solubility of hydrogen sulfide and carbon dioxide in aqueous methyldiethanolamine solutions. Ind Eng Chem Proc Des Dev 21:539–544. https://doi.org/10.1021/i200019a001
Jou FY, Carroll JJ, Mather AE, Otto FD (1993a) Solubility of mixtures of hydrogen sulfide and carbon dioxide in aqueous N-methyldiethanolamine solutions. J Chem Eng Data 38:75–77. https://doi.org/10.1021/je00009a018
Jou FY, Carroll JJ, Mather AE, Otto FD (1993b) The solubility of carbon dioxide and hydrogen sulfide in a 35 wt% aqueous solution of methyldiethanolamine. Can J Chem Eng 71:264–268. https://doi.org/10.1002/cjce.5450710213
Kamps APS, Balaban A, Jodecke M, Kuranov G, Smirnova NA, Maurer G (2001) Solubility of single gases carbon dioxide and hydrogen sulfide in aqueous solutions of N-mythyldiethanolamine at temperatures from 313 to 393 K and pressures up to 7.6 MPa: new experimental data and model extension. Ind Eng Chem Res 40:696–706. https://doi.org/10.1021/ie000441r
Kenig EY, Schneider R, Gorak A (2001) Reactive absorption: optimal process design via optimal modeling. Chem Eng Sci 56:343–350. https://doi.org/10.1016/S0009-2509(00)00234-7
Kim I, Svendsen HF, Borresen E (2008) Ebulliometric determination of vapor-liquid equilibria for pure water, monoethanolamine, N-methyldiethanolamine, 3-(methylamino)-propylamine, and their binary and ternary solutions. J Chem Eng Data 53:2521–2531. https://doi.org/10.1021/je800290k
Klöker M, Kenig EY, Hoffmann A, Kreis P, Górak A (2005) Rate-based modelling and simulation of reactive separations in gas/vapour–liquid systems. Chem Eng Process Process Intensif 44:617–629. https://doi.org/10.1016/j.cep.2003.12.011
Kopac T (2021a) Hydrogen storage characteristics of bio-based porous carbons of different origin: a comparative review. Int J Energy Res 45:20497–20523. https://doi.org/10.1002/er.7130
Kopac T (2021b) Emerging applications of process intensification for enhanced separation and energy efficiency, environmentally friendly sustainable adsorptive separations: a review. Int J Energy Res 45:15839–15856. https://doi.org/10.1002/er.6944
Kopac T (2023) Current overview of the valorization of bio-wastes for adsorbed natural gas applications. Carbon Lett 33:1519–1547. https://doi.org/10.1007/s42823-023-00508-0
Kothandaraman A (2010) Carbon dioxide capture by chemical absorption: a solvent comparison study. PhD thesis, Massachusetts Institute of Technology, Boston
Kucka L, Müller I, Kenig EY, Górak A (2003) On the modeling and simulation of sour gas absorption by aqueous amine solutions. Chem Eng Sci 58:3571–3578. https://doi.org/10.1016/S0009-2509(03)00255-0
Kundu M, Mandal BP, Bandyopadhyay S (2003) Vapor-liquid equilibrium of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol. J Chem Eng Data 48:789–796. https://doi.org/10.1021/je0201626
Kuranov G, Rumpf B, Smirnova NA, Maurer G (1996) Solubility of single gases carbon dioxide and hydrogen sulfide in aqueous solutions of N-methyldiethanolamine in the temperature range 313–413 K at pressures up to 5 MPa. Ind Eng Chem Res 35:1959–1966. https://doi.org/10.1021/ie950538r
Lawson JD, Garst AW (1976) Gas sweetening data: equilibrium solubility of hydrogen sulfide and carbon dioxide in aqueous monoethanolamine and aqueous diethanolamine solutions. J Chem Eng Data 21:20–30. https://doi.org/10.1021/je60068a010
Li MH, Chang BC (1994) Solubilities of carbon dioxide in water + monoethanolamine + 2-amino-2-methyl-1-propanol. J Chem Eng Data 39:448–452. https://doi.org/10.1021/je00015a010
Lungkadee T, Onsree T, Tangparitkul S, Janwiruch N, Nuntaphan A, Tippayawong N (2021) Technical and economic analysis of retrofitting a post-combustion carbon capture system in a Thai coal-fired power plant. Energy Rep 7:308–313. https://doi.org/10.1016/j.egyr.2021.06.049
Luo X, Liu S, Gao H, Liao H, Tontiwachwuthikul P, Liang Z (2016) An improved fast screening method for single and blended amine-based solvents for post-combustion CO2 capture. Sep Purif Technol 169:279–288. https://doi.org/10.1016/j.seppur.2016.06.018
Ma’mun S, Svendsen HF, Karl AH, Olav J (2007) Selection of new absorbents for carbon dioxide capture. Energy Convers Manag 48:251–258. https://doi.org/10.1016/j.enconman.2006.04.007
Maddox RN, Elizondo EM (1989) Equilibrium solubility of carbon dioxide or hydrogen sulfide in aqueous solutions of diethanolamine at low partial pressures. GPA Research Report, No. 124
Maddox RN, Bhairl AH, Diers JR, Thomas PA (1987) Equilibrium solubility of carbon dioxide or hydrogen sulfide in aqueous solutions of monoethanolamine, diglycolamine, diethanolamine and methldiethanolamine. GPA Research Report, No. 104
Madejski P, Chmiel K, Subramanian N, Kuś T (2022) Methods and techniques for CO2 capture: review of potential solutions and applications in modern energy technologies. Energies 15:887. https://doi.org/10.3390/en15030887
Maham Y, Mather AE, Hepler LG (1997) Excess molar enthalpies of (water + alkanolamine) systems and some thermodynamic calculations. J Chem Eng Data 42:988–992. https://doi.org/10.1021/je960296h
Maham Y, Mather AE, Mathonat C (2000) Excess properties of (alkyldiethanolamine + H2O) mixtures at temperatures from (298.15 to 338.15) K. J Chem Thermodyn 32:229–236. https://doi.org/10.1006/jcht.1999.0595
Manzolini G, Giuffrida A, Cobden PD, van Dijk HAJ, Ruggeri F, Consonni F (2020) Techno-economic assessment of SEWGS technology when applied to integrated steel-plant for CO2 emission mitigation. Int J Greenh Gas Control 94:102935. https://doi.org/10.1016/j.ijggc.2019.102935
Mathonat C, Maham Y, Mather AE, Hepler LG (1997) Excess molar enthalpies of (water + monoalkanolamine) mixtures at 298.15K and 308.15K. J Chem Eng Data 42:993–995. https://doi.org/10.1021/je960304u
Mathonat C (1995) Calorimetrie de mélange, a ecoulement, a temperatures et pressions elevees. Application a l’etude de l’elimination du dioxide de carbone a l’aide de solutions aqueuses d’alcanolamines. PhD Thesis, Universite Blaise Pascal
Mock B, Evans LB, Chen CC (1986) Thermodynamic representation of phase equilibria of mixed-solvent electrolyte systems. AIChE J 32:1655–1664. https://doi.org/10.1002/aic.690321009
Mostafavi E, Ashrafi O, Navarri P (2021) Assessment of process modifications for amine-based post-combustion carbon capture processes. Clean Eng Technol 4:100249. https://doi.org/10.1016/j.clet.2021.100249
Nesterenko N, Medeiros-Costa IC, Clatworthy EB, Cruchade H, Konnov SV, Dath JP, Gilson JP, Mintova S (2023) Methane-to-chemicals: a pathway to decarbonization. Natl Sci Rev 10(9):nwad116. https://doi.org/10.1093/nsr/nwad116
Nielsen CJ, Herrmann H, Weller C (2012) Atmospheric chemistry and environmental impact of the use of amines in carbon capture and storage (CCS). Chem Soc Rev 41:6684–6704. https://doi.org/10.1039/C2CS35059A
Nord L, Bolland O (2020) Carbon dioxide emission management in power generation. Wiley-VCH Verlag GmbH & Co., Weinheim, Germany
Oexmann J, Hensel C, Kather A (2008) Post-combustion CO2-capture from coal-fired power plants: preliminary evaluation of an integrated chemical absorption process with piperazine-promoted potassium carbonate. Int J Greenh Gas Control 2:539–552. https://doi.org/10.1016/j.ijggc.2008.04.002
Otitoju O, Oko E, Wang M (2021) Technical and economic performance assessment of post-combustion carbon capture using piperazine for large scale natural gas combined cycle power plants through process simulation. Appl Energy 292:116893. https://doi.org/10.1016/j.egyr.2021.06.049
Pappa GD, Anastasi C, Voutsas EC (2006) Measurement and thermodynamic modeling of the phase equilibrium of aqueous 2-amino-2-methyl-1-propanol solutions. Fluid Phase Equilib 243:193–197. https://doi.org/10.1016/j.fluid.2006.03.014
Pellegrini G, Strube R, Manfrida G (2010) Comparative study of chemical absorbents in postcombustion CO2 capture. Energy 35:851–857. https://doi.org/10.1016/j.energy.2009.08.011
Pinsent BR, Pearson L, Roughton FJW (1956) The kinetics of combination of carbon dioxide with hydroxide ions. Trans Faraday Soc 52:1512–1520. https://doi.org/10.1039/TF9565201512
Pitzer KS (1980) Electrolytes. From dilute solutions to fused salts. J Am Chem Soc 102(9):2902–2906. https://doi.org/10.1021/ja00529a006
Posey ML (1996) Thermodynamic model for acid gas loaded aqueous alkanolamine solutions. PhD Thesis, The University of Texas at Austin
Repke J, Villain O, Wozny G (2004) A nonequilibrium model for three-phase distillation in a packed column: modeling and experiments. Comput Chem Eng 28:775–780. https://doi.org/10.1016/j.compchemeng.2004.02.016
Rinker EB, Ashour SS, Sandall OC (1996) Kinetics and modeling of carbon dioxide absorption into aqueous solutions of diethanolamine. Ind Eng Chem Res 35:1107–1114. https://doi.org/10.1021/ie950336v
Rinker EB, Ashour SS, Sandall OC (1997) Experimental absorption rate measurements and reaction kinetics for H2S and CO2 in aqueous DEA, MDEA and blends of DEA and MDEA, GPA Research Report, No. 159
Rissman J, Bataille C, Masanet E, Aden N, Morrow WR, Zhou N, Elliott N, Dell R, Heeren N, Huckestein B et al (2020) Technologies and policies to decarbonize global industry: review and assessment of mitigation drivers through 2070. Appl Energy 266:114848. https://doi.org/10.1016/j.apenergy.2020.114848
Roberts BE, Mather AE (1988) Solubility of CO2 and H2S in a hindered amine solution. Chem Eng Comm 64:105–111. https://doi.org/10.1080/00986448808940230
Robinson RA, Stokes RH (1970) Electrolyte solutions. Butterworths, London
Rochelle GT (2009) Amine scrubbing for CO2 capture. Science 325(5948):1652–1654. https://doi.org/10.1126/science.1176731
Rockström J, Gaffney O, Rogelj J, Meinshausen M, Nakicenovic N, Schellnhuber HJ (2017) A roadmap for rapid decarbonization. Science 355(6331):1269–1271. https://doi.org/10.1126/science.aah3443
Seo DJ, Hong WH (1992) Solubilities of carbon dioxide in aqueous mixtures of diethanolamine and 2-amino-2-methyl-1-propanol. J Chem Eng Data 41:258–260. https://doi.org/10.1021/je950197o
Springer PAM, van der Molen S, Krishna R (2002) The need for using rigorous rate-based models for simulations of ternary azeotropic distillation. Comput Chem Eng 26:1265–1279. https://doi.org/10.1016/S0098-1354(02)00039-X
Sreedhar I, Vaidhiswaran R, Kamani BM, Venugopal A (2017a) Process and engineering trends in membrane based carbon capture. Renew Sust Energ Rev 68:659–684. https://doi.org/10.1016/j.rser.2016.10.025
Sreedhar I, Nahar T, Venugopal A, Srinivas B (2017b) Carbon capture by absorption – path covered and ahead. Renew Sust Energ Rev 76:1080–1107. https://doi.org/10.1016/j.rser.2017.03.109
Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM, Biggs R, Carpenter SR, De Vries W, De Wit CA et al (2015) Planetary boundaries: guiding human development on a changing planet. Science 347:1259855. https://doi.org/10.1126/science.1259855
Succar SA, Brissaud D, Evrard D, Flick D, De la Fontaine D (2023) Decarbonization measure: a concept towards the acceleration of the automotive plant decarbonization. Systems 11(7):335. https://doi.org/10.3390/systems11070335
Sultan H, Bhatti UH, Muhammad HA, Nam SC, Baek IH (2021) Modification of postcombustion CO2 capture process: a techno-economic analysis. Greenhouse Gas Sci Technol 11:165–182. https://doi.org/10.1002/ghg.2042
Sundaramoorthy S, Kamath D, Nimbalkar S, Price C, Wenning T, Cresko J (2023) Energy efficiency as a foundational technology pillar for industrial decarbonization. Sustainability 15(12):9487. https://doi.org/10.3390/su15129487
Takenouchi S, Kennedy GC (1964) The binary system H2O–CO2 at high temperatures and pressures. Am J Sci 262:1055–1074. https://doi.org/10.2475/ajs.262.9.1055
Taylor R, Krishna R, Kooijman H (2003) Real-world modeling of distillation. Chem Eng Prog 99(7):28–39
Teng TT, Mather AE (1990) J Chem Eng Data 35(4):410–411. https://doi.org/10.1021/je00062a010
Tödheide K, Franck EU (1963) Das Zweiphasengebiet und die Kritische Kurve im System Kohlendioxid-Wasser bis zu Drucken von 3500 bar Zeitschrift fur Physikalische Chemie Neue Folge, BD. 37, S.387–401. https://doi.org/10.1524/zpch.1963.37.5_6.387
Tontiwachwuthikul P, Meisen A, Lim CJ (1991) Solubility of CO2 in 2-amino-2-methyl-1-propanol solutions. J Chem Eng Data 36:130–133. https://doi.org/10.1021/je00001a038
Torralba-Calleja E, Skinner J, Gutiérrez-Tauste D (2013) CO2 capture in ionic liquids: a review of solubilities and experimental methods. J Chem 2013:473584. https://doi.org/10.1155/2013/473584
Treybal RE (1981) Mass-transfer operations. Chemical Engineering Series, McGraw-Hill, Auckland
UNFCCC (2023) United Nations Framework Convention on Climate Change The Paris Agreement. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement. Accessed on 15 May 2023
Varghese AM, Karanikolos GN (2020) CO2 capture adsorbents functionalized by amine – bearing polymers: a review. Int J Greenh Gas Control 96:103005. https://doi.org/10.1016/j.ijggc.2020.103005
Varghese AM, Reddy KSK, Singh S, Karanikolos GN (2020) Performance enhancement of CO2 capture adsorbents by UV treatment: the case of self-supported graphene oxide foam. Chem Eng J 386:124022. https://doi.org/10.1016/j.cej.2020.124022
Voldsund M, Gardarsdottir SO, De Lena E, Pérez-Calvo JF, Jamali A, Berstad D, Fu C, Romano M, Roussanaly S, Anantharaman R et al (2019) Comparison of technologies for CO2 capture from cement production—part 1: technical evaluation. Energies 12:559. https://doi.org/10.3390/en12030559
Voutsas E, Vrachnos A, Magoulas K (2004) Measurement and thermodynamic modeling of the phase equilibrium of aqueous N-methyldiethanolamine solutions. Fluid Phase Equilib 224:193–197. https://doi.org/10.1016/j.fluid.2004.05.012
Wang YW, Xu S, Otto FD, Mather AE (1992) Solubility of N2O in alkanolamines and in mixed solvents. Chem Eng J 48:31–40. https://doi.org/10.1016/0300-9467(92)85004-S
Wang R, Li DF, Liang DT (2004) Modeling of CO2 capture by three typical amine solutions in hollow fiber membrane contactors. Chem Eng Process: Process Intensif 43(7):849–856. https://doi.org/10.1016/S0255-2701(03)00105-3
Wang Y, Zhao L, Otto A, Robinius M, Stolten D (2017) A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Procedia 114:650–665. https://doi.org/10.1016/j.egypro.2017.03.1209
Weiland RH, Dingman JC, Cronin DB (1997) Heat capacity of aqueous monoethanolamine, diethanolamine, N-methyldiethanolamine, and N-methyldiethanolamine-based blends with carbon dioxide. J Chem Eng Data 42:1004–1006. https://doi.org/10.1021/je960314v
Weiland RH, Dingman JC, Cronin DB, Browning GJ (1998) Density and viscosity of some partially carbonated aqueous alkanolamine solutions and their blends. J Chem Eng Data 43:378–382. https://doi.org/10.1021/je9702044
Weiland RH (1996) Physical properties of MEA, DEA, MDEA and MDEA-based blends loaded with CO2, GPA Research Report, No. 152
Wiebe R, Gaddy VL (1940) The solubility of carbon dioxide in water at various temperatures from 12 to 40 °C and at pressures to 500 atm. Critical Phenomena J Am Chem Soc 62:815–817. https://doi.org/10.1021/ja01861a033
Xu S, Qing S, Zhen Z, Zhang C, Carroll J (1991) Vapor pressure measurements of aqueous N-methyldiethanolamine solutions. Fluid Phase Equilib 67:197–201. https://doi.org/10.1016/0378-3812(91)90055-C
Yu K, Curcic I, Gabriel J, Tsang S (2008) Recent advances in CO2 capture and utilization. Chemsuschem 1:893–899. https://doi.org/10.1002/cssc.200800169
Zawisza A, Malesiska B (1981) Solubility of carbon dioxide in liquid water and of water in gaseous carbon dioxide in the range 0.2-5 MPa and at temperature up to 473K. J Chem Eng Data 26:388–391. https://doi.org/10.1021/je00026a012
Zhang K, Hawrylak B, Palepu R, Tremaine PR (2002) Thermodynamics of aqueous amines: excess molar heat capacities, volumes, and expansibilities of (water + methyldiethanolamine (MDEA)) and (water + 2-amino-2-methyl-1-propanol (AMP)). J Chem Thermodyn 34:679–710. https://doi.org/10.1006/jcht.2002.0937
Zhang Y, Chen H, Chen CC, Plaza JM, Dugas R, Rochelle GT (2009) Rate-based process modeling study of CO2 capture with aqueous monoethanolamine solution. Ind Eng Chem Res 48:9233–9246. https://doi.org/10.1021/ie900068k
Zheng S, Jin S (2023) Can companies reduce carbon emission intensity to enhance sustainability? Systems 11(5):249. https://doi.org/10.3390/systems11050249
Zhou Q, Chan CW, Tontiwachwuthikul P (2011) An intelligent system for monitoring and diagnosis of the CO2 capture process. Expert Syst Appl 38(7):7935–7946. https://doi.org/10.1016/j.eswa.2010.12.010
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, modeling, and analysis were performed by Turkan Kopac and Yaşar Demirel. The first draft of the manuscript was written by Turkan Kopac and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kopac, T., Demirel, Y. Impact of thermodynamics and kinetics on the carbon capture performance of the amine-based CO2 capture system. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33792-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-33792-y