Abstract
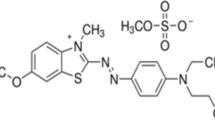
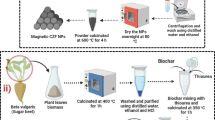
In the printing and textile industries, methylene blue (a cationic azo dye) is commonly used. MB is a well-known carcinogen, and another major issue is its high content in industrial discharge. There are numerous removal methodologies that have been employed to remove it from industrial discharge; however, these current modalities have one or more limitations. In this research, a novel magnetized biochar (γ-Fe2O3-LSB) was synthesized using Lagenaria siceraria peels which were further magnetized via the co-precipitation method. The synthesized γ-Fe2O3-LSB was characterized using FTIR, X-ray diffraction, Raman, SEM–EDX, BET, and vibrating sample magnetometry (VSM) for the analysis of magnetic properties. γ-Fe2O3-LSB showed a reversible type IV isotherm, which is a primary characteristic of mesoporous materials. γ-Fe2O3-LSB had a specific surface area (SBET = 135.30 m2/g) which is greater than that of LSB (SBET = 11.54 m2/g). γ-Fe2O3-LSB exhibits a saturation magnetization value (Ms) of 3.72 emu/g which shows its superparamagnetic nature. The batch adsorption process was performed to analyze the adsorptive removal of MB dye using γ-Fe2O3-LSB. The adsorption efficiency of γ-Fe2O3-LSB for MB was analyzed by varying parameters like the initial concentration of adsorbate (MB), γ-Fe2O3-LSB dose, pH effect, contact time, and temperature. Adsorption isotherm, kinetic, and thermodynamics were also studied after optimizing the protocol. The non-linear Langmuir model fitted the best to explain the adsorption isotherm mechanism and resulting adsorption capacity (\({q}_{e}\) =54.55 mg/g). The thermodynamics study showed the spontaneous and endothermic nature, and pseudo-second-order rate kinetics was followed during the adsorption process. Regeneration study showed that γ-Fe2O3-LSB can be used up to four cycles. In laboratory setup, the cost of γ-Fe2O3-LSB synthesis comes out to be 162.75 INR/kg which is low as compared to commercially available adsorbents. The results obtained suggest that magnetic Lagenaria siceraria biochar, which is economical and efficient, can be used as a potential biochar material for industrial applications in the treatment of wastewater.
Graphical Abstract


















Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
A.O D (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn 2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem 3:38–45.https://doi.org/10.9790/5736-0313845
Adams MJ, Antoniw JF, Bar-Joseph M et al (2004) Virology division news: the new plant virus family Flexiviridae and assessment of molecular criteria for species demarcation. Arch Virol 149:1045–1060. https://doi.org/10.1007/s00705-004-0304-0
Agarwal S, Sadegh H, Monajjemi M et al (2016) Efficient removal of toxic bromothymol blue and methylene blue from wastewater by polyvinyl alcohol. J Mol Liq 218:191–197. https://doi.org/10.1016/j.molliq.2016.02.060
Ahmed MJ, Okoye PU, Hummadi EH, Hameed BH (2019) High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour Technol 278:159–164. https://doi.org/10.1016/j.biortech.2019.01.054
Ahmed S, Al-mamun R, Ali MA et al (2023) Jute stick-derived cellulose-based hydrogel: synthesis, characterization, and methylene blue removal from aqueous solution. https://doi.org/10.1021/acsomega.3c06349
Ai T, Jiang X, Liu Q et al (2019) Daptomycin adsorption on magnetic ultra-fine wood-based biochars from water: kinetics, isotherms, and mechanism studies. Bioresour Technol 273:8–15. https://doi.org/10.1016/j.biortech.2018.10.039
Ai L, Zhang C, Liao F et al (2011) Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J Hazard Mater 198. https://doi.org/10.1016/j.jhazmat.2011.10.041
Ajinkya N, Yu X, Kaithal P et al (2020) Magnetic iron oxide nanoparticle (Ionp) synthesis to applications: present and future. Materials 13:1–35. https://doi.org/10.3390/ma13204644
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383. https://doi.org/10.1016/j.jhazmat.2020.122383
Al-Qodah Z, Lafi WK, Al-Anber Z et al (2007) Adsorption of methylene blue by acid and heat treated diatomaceous silica. Desalination 217:212–224. https://doi.org/10.1016/j.desal.2007.03.003
Al-Qodah Z, Dweiri R, Khader M, Al-Sabbagh S, Al-Shannag M, Qasrawi S, Al-Halawani M (2023) Processing and characterization of magnetic composites of activated carbon, fly ash, and beach sand as adsorbents for Cr(VI) removal. Case Studies Chem Environ Eng 7:100333. https://doi.org/10.1016/j.cscee.2023.100333
Altıntıg E, Altundag H, Tuzen M et al (2017) Effective removal of methylene blue from aqueous solutions using magnetic loaded activated carbon as novel adsorbent. Chem Eng Res Des 122:151–163. https://doi.org/10.1016/j.cherd.2017.03.035
Amin MT, Alazba AA, Shafiq M (2019) Comparative study for adsorption of methylene blue dye on biochar derived from orange peel and banana biomass in aqueous solutions. Environ Monit Assess 191. https://doi.org/10.1007/s10661-019-7915-0
Bayomie OS, Kandeel H, Shoeib T et al (2020) Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-64727-5
Boudraa I, Odabasi SU, Bareera M et al (2022) Magnetization of a biochar derived from orange peel and its application for the removal of crystal violet. Appl Environ Res 44:88–100. https://doi.org/10.35762/AER.2022.44.3.7
Burbano AA, Gascó G, Horst F, et al (2023) Production, characteristics and use of magnetic biochar nanocomposites as sorbents. Biomass Bioenergy 172. https://doi.org/10.1016/j.biombioe.2023.106772
Cai T, Liu X, Zhang J et al (2021) Silicate-modified oiltea camellia shell-derived biochar: a novel and cost-effective sorbent for cadmium removal. J Clean Prod 281. https://doi.org/10.1016/j.jclepro.2020.125390
Chang J, Yu S, Liao Y et al (2022) One-step pyrolysis fabrication of magnetic bagasse biochar composites with excellent lead adsorption performance. ACS Omega 7:42854–42864. https://doi.org/10.1021/acsomega.2c04882
Chen Z, Zhang J, Fu J et al (2014) Adsorption of methylene blue onto poly(cyclotriphosphazene-co-4,4’-sulfonyldiphenol) nanotubes: kinetics, isotherm and thermodynamics analysis. J Hazard Mater 273:263–271. https://doi.org/10.1016/j.jhazmat.2014.03.053
Cheng L, Ji Y (2024) Photocatalytic activation of sulfite by N-doped porous biochar/MnFe2O4 interface-driven catalyst for efficient degradation of tetracycline. Green Energy Environ 9:481–494. https://doi.org/10.1016/j.gee.2022.07.006
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168. https://doi.org/10.1016/j.desal.2010.07.047
Chung K-T (2016) ACCEPTED MANUSCRIPT ACCEPTED MANUSCRIPT Azo dyes and human health: a review. Environ Sci Health Care 34:233–261
Dao MU, Le HS, Hoang HY et al (2021) Natural core-shell structure activated carbon beads derived from Litsea glutinosa seeds for removal of methylene blue: facile preparation, characterization, and adsorption properties. Environ Res 198:110481. https://doi.org/10.1016/j.envres.2020.110481
Dobrzyńska J, Wysokińska A, Olchowski R (2022) Raspberry stalks-derived biochar, magnetic biochar and urea modified magnetic biochar - synthesis, characterization and application for As(V) and Cr(VI) removal from river water. J Environ Manage 316. https://doi.org/10.1016/j.jenvman.2022.115260
Dos Reis GS, de Oliveira HP, Larsson SH et al (2021) A short review on the electrochemical performance of hierarchical and nitrogen-doped activated biocarbon-based electrodes for supercapacitors. Nanomaterials 11:1–17. https://doi.org/10.3390/nano11020424
El-Nemr MA, Abdelmonem NM, Ismail IMA et al (2020) The efficient removal of the hazardous azo dye acid orange 7 from water using modified biochar from pea-peels. Desalin Water Treat 203:327–355. https://doi.org/10.5004/dwt.2020.26190
Feng Y, Liu Y, Xue L et al (2017) Bioresource Technology Carboxylic acid functionalized sesame straw : a sustainable cost-effective bioadsorbent with superior dye adsorption capacity. Bioresour Technol 238:675–683. https://doi.org/10.1016/j.biortech.2017.04.066
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Hoslett J, Ghazal H, Mohamad N, Jouhara H (2020) Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci Total Environ 714. https://doi.org/10.1016/j.scitotenv.2020.136832
Jawad AH, Saud Abdulhameed A, Wilson LD et al (2021) High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: Optimization and mechanism study. Chin J Chem Eng 32:281–290. https://doi.org/10.1016/j.cjche.2020.09.070
Kadhom M, Albayati N, Alalwan H, Al-Furaiji M (2020) Removal of dyes by agricultural waste. Sustain Chem Pharm 16. https://doi.org/10.1016/j.scp.2020.100259
Kapoor RT, Rafatullah M, Aljuwayid AM et al (2022) Removal of patent blue dye using ananas comosus-derived biochar: equilibrium, kinetics, and phytotoxicity studies. Separations 9. https://doi.org/10.3390/separations9120426
Kong X, Liu Y, Pi J et al (2017) Low-cost magnetic herbal biochar: characterization and application for antibiotic removal. Environ Sci Pollut Res 24:6679–6687. https://doi.org/10.1007/s11356-017-8376-z
Kumar NS, Shaikh HM, Asif M, Al-Ghurabi EH (2021) Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Sci Rep 11. https://doi.org/10.1038/s41598-021-82277-2
Kundu S, Gupta AK (2006) Arsenic adsorption onto iron oxide-coated cement ( IOCC ): Regression analysis of equilibrium data with several isotherm models and their optimization 122:93–106. https://doi.org/10.1016/j.cej.2006.06.002
Li Y, Zimmerman AR, He F et al (2020) Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue. Sci Total Environ 722:137972. https://doi.org/10.1016/j.scitotenv.2020.137972
Li C, Zhang C, Zhong S et al (2023) The removal of pollutants from wastewater using magnetic biochar: a scientometric and visualization analysis. Molecules 28:1–24. https://doi.org/10.3390/molecules28155840
Liu S, Li J, Xu S et al (2019) A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour Technol 282:48–55. https://doi.org/10.1016/j.biortech.2019.02.092
Liu Y, Liu Y (2008) Biosorption isotherms, kinetics and thermodynamics 61:229–242. https://doi.org/10.1016/j.seppur.2007.10.002
Ma Y, Qi Y, Yang L et al (2021) Adsorptive removal of imidacloprid by potassium hydroxide activated magnetic sugarcane bagasse biochar: adsorption efficiency, mechanism and regeneration. J Clean Prod 292. https://doi.org/10.1016/j.jclepro.2021.126005
Mahmoud DK, Salleh MAM, Karim WAWA et al (2012) Batch adsorption of basic dye using acid treated kenaf fibre char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 181–182:449–457. https://doi.org/10.1016/j.cej.2011.11.116
Majamo SL, Amibo TA, Bedru TK (2023) Synthesis and application of biomass-derived magnetic biochar catalyst for simultaneous esterification and trans-esterification of waste cooking oil into biodiesel: modeling and optimization. Mater Renew Sustain Energy 12:147–158. https://doi.org/10.1007/s40243-023-00236-5
Mansee AH, Abdelgawad DM, Gamal EH El et al (2023) Influences of Mg - activation on sugarcane bagasse biochar characteristics and its PNP removing potentials from contaminated water. Sci Rep 1–17. https://doi.org/10.1038/s41598-023-46463-8
Mishra A, Ojha H, Pandey J, et al (2023) Adsorption characteristics of magnetized biochar derived from Citrus limetta peels. Heliyon 9. https://doi.org/10.1016/j.heliyon.2023.e20665
Misran E, Bani O, Situmeang EM, Purba AS (2022) Banana stem based activated carbon as a low-cost adsorbent for methylene blue removal: isotherm, kinetics, and reusability. Alex Eng J 61:1946–1955. https://doi.org/10.1016/j.aej.2021.07.022
Mo H, Qiu J (2020) Preparation of chitosan/magnetic porous biochar as support for cellulase immobilization by using glutaraldehyde. Polymers (basel) 12:1–14. https://doi.org/10.3390/polym12112672
Muralikrishnan R, Jodhi C (2020) Biodecolorization of reactive dyes using biochar derived from coconut shell: batch, isotherm, kinetic and desorption studies. ChemistrySelect 5:7734–7742. https://doi.org/10.1002/slct.202001454
Ngernyen Y, Petsri D, Sribanthao K et al (2023) Adsorption of the non-steroidal anti-inflammatory drug (ibuprofen) onto biochar and magnetic biochar prepared from chrysanthemum waste of the beverage industry. RSC Adv 13:14712–14728. https://doi.org/10.1039/d3ra01949g
NITI Aayog (2018) Composite water management index. https://social.niti.gov.in/uploads/sample/water_index_report.pdf
Oliveira LCA, Rios RVRA, Fabris JD et al (2002) Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon N Y 40:2177–2183. https://doi.org/10.1016/S0008-6223(02)00076-3
Radoor S, Karayil J, Jayakumar A et al (2022) Ecofriendly and low-cost bio adsorbent for efficient removal of methylene blue from aqueous solution. Sci Rep 12. https://doi.org/10.1038/s41598-022-22936-0
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80. https://doi.org/10.1016/j.jhazmat.2009.12.047
Rasoulpoor K, Poursattar Marjani A, Nozad E (2020) Competitive chemisorption and physisorption processes of a walnut shell based semi-IPN bio-composite adsorbent for lead ion removal from water: equilibrium, kinetic and thermodynamic studies. Environ Technol Innov 20:101133. https://doi.org/10.1016/j.eti.2020.101133
Robati D, Mirza B, Ghazisaeidi R et al (2016) Adsorption behavior of methylene blue dye on nanocomposite multi-walled carbon nanotube functionalized thiol (MWCNT-SH) as new adsorbent. J Mol Liq 216:830–835. https://doi.org/10.1016/j.molliq.2016.02.004
Saha P, Chowdhury S (2011) Insight into adsorption thermodynamics. Thermodynamics. https://doi.org/10.5772/13474
Salem DB, Ouakouak A, Touahra F et al (2023) Easy separable, floatable, and recyclable magnetic-biochar/alginate bead as super-adsorbent for adsorbing copper ions in water media. Bioresour Technol 383:129225. https://doi.org/10.1016/j.biortech.2023.129225
Sayed NSM, Ahmed ASA, Abdallah MH, Gouda GA (2024) ZnO@ activated carbon derived from wood sawdust as adsorbent for removal of methyl red and methyl orange from aqueous solutions. Sci Rep 14:1–18. https://doi.org/10.1038/s41598-024-55158-7
Selvarajoo A, Oochit D (2020) Effect of pyrolysis temperature on product yields of palm fibre and its biochar characteristics. Mater Sci Energy Technol 3:575–583. https://doi.org/10.1016/j.mset.2020.06.003
Sewu DD, Boakye P, Woo SH (2017) Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Bioresour Technol 224:206–213. https://doi.org/10.1016/j.biortech.2016.11.009
Shahryari Z, Goharrizi AS, Azadi M (2010) Experimental study of methylene blue adsorption from aqueous solutions onto carbon nano tubes. Int J Water Resour Environ Eng 2(2):16–28
Shao Q, Li Y, Wang Q et al (2021) Preparation of copper doped walnut shell-based biochar for efficiently removal of organic dyes from aqueous solutions. J Mol Liq 336:116314. https://doi.org/10.1016/j.molliq.2021.116314
Sun P, Hui C, Khan RA et al (2015) Efficient removal of crystal violet using Fe3O4-coated biochar: The role of the Fe3O4 nanoparticles and modeling study their adsorption behavior. Sci Rep 5. https://doi.org/10.1038/srep12638
Talebi SS, Javid AB, Roudbari AA et al (2021) Defluoridation of drinking water by metal impregnated multi-layer green graphene fabricated from trees pruning waste. Environ Sci Pollut Res 28:18201–18215. https://doi.org/10.1007/s11356-020-11743-7
Tan X, Liu Y, Zeng G et al (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85. https://doi.org/10.1016/j.chemosphere.2014.12.058
Tran HN, You SJ, Chao HP (2016) Effect of pyrolysis temperatures and times on the adsorption of cadmium onto orange peel derived biochar. Waste Manag Res 34:129–138. https://doi.org/10.1177/0734242X15615698
Verma V, Chandra Y (2023) Facile preparation, characterization and application of novel sugarcane bagasse–derived nanoceria-biochar for defluoridation of drinking water: kinetics, thermodynamics, reusability and mechanism. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-30993-9
Vigneshwaran S, Sirajudheen P, Karthikeyan P, Meenakshi S (2021) Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of Malachite green and Rhodamine B dyes. Surf Interfaces 23:100920. https://doi.org/10.1016/j.surfin.2020.100920
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel ( II ) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models 133:304–308. https://doi.org/10.1016/j.jhazmat.2005.10.016
Vyavahare GD, Gurav RG, Jadhav PP et al (2018) Response surface methodology optimization for sorption of malachite green dye on sugarcane bagasse biochar and evaluating the residual dye for phyto and cytogenotoxicity. Chemosphere 194:306–315. https://doi.org/10.1016/j.chemosphere.2017.11.180
Wang Y, Mu Y, Zhao QB, Yu HQ (2006) Isotherms, kinetics and thermodynamics of dye biosorption by anaerobic sludge. Sep Purif Technol 50:1–7. https://doi.org/10.1016/j.seppur.2005.10.012
Wang H, Wang H, Zhao H, Yan Q (2020) Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu blue algae. Chem Eng J 379. https://doi.org/10.1016/j.cej.2019.122372
Yahya MA, Zanariah CW, Ngah CW, Hashim MA, Al-Qodah Z (2015) Preparation of activated carbon from desiccated coconut residue by chemical activation with NaOH. J Mater Sci Res 5:24. https://doi.org/10.5539/jmsr.v5n1p24
Yin Z, Liu Y, Liu S et al (2018) Activated magnetic biochar by one-step synthesis: enhanced adsorption and coadsorption for 17β-estradiol and copper. Sci Total Environ 639:1530–1542. https://doi.org/10.1016/j.scitotenv.2018.05.130
Zahedifar M, Seyedi N (2022) Bare 3D-TiO2/magnetic biochar dots (3D-TiO2/BCDs MNPs): Highly efficient recyclable photocatalyst for diazinon degradation under sunlight irradiation. Phys E Low Dimens Syst Nanostruct 139. https://doi.org/10.1016/j.physe.2022.115151
Zeghioud H, Mouhamadou S (2023) Easy recovered magnetic bark biochar for methylene blue removal: preparation characterization and adsorption parameters study. ChemistrySelect 8. https://doi.org/10.1002/slct.202302788
Zeghioud H, Mouhamadou S (2023a) Dye removal characteristics of magnetic biochar derived from sewage sludge: isotherm, thermodynamics, kinetics, and mechanism. Water Air Soil Pollut 234. https://doi.org/10.1007/s11270-023-06251-6
Zhang P, Lo I, O’Connor D et al (2017) High efficiency removal of methylene blue using SDS surface-modified ZnFe2O4 nanoparticles. J Colloid Interface Sci 508:39–48. https://doi.org/10.1016/j.jcis.2017.08.025
Zhang X, Lv L, Qin Y et al (2018) Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour Technol 256:1–10. https://doi.org/10.1016/j.biortech.2018.01.145
Zhang Z, Zhu Z, Shen B, Liu L (2019) Insights into biochar and hydrochar production and applications: A review. Energy 171:581–598
Zhang L, Ren Y, Xue Y et al (2020a) Preparation of biochar by mango peel and its adsorption characteristics of Cd(ii) in solution. RSC Adv 10:35878–35888. https://doi.org/10.1039/d0ra06586b
Zhang P, O’Connor D, Wang Y et al (2020b) A green biochar/iron oxide composite for methylene blue removal. J Hazard Mater 384:121286. https://doi.org/10.1016/j.jhazmat.2019.121286
Zhang Z, Zhou C, Yang J et al (2022) Preparation and characterization of apricot kernel shell biochar and its adsorption mechanism for atrazine. Sustainability (switzerland) 14:1–15. https://doi.org/10.3390/su14074082
Zhao T, Chen R, Wang J (2020) A mild method for preparation of highly selective magnetic biochar microspheres. Int J Mol Sci 21. https://doi.org/10.3390/ijms21113752
Acknowledgements
All authors are sincerely thankful to the Director of the Institute of Nuclear Medicine and Allied Sciences (INMAS) for supporting this research work. The authors are grateful to Dr. Anjani Kumar Tiwari, Head, Department of Chemistry, Babasaheb Bhimrao Ambedkar University, Lucknow, for his guidance and to Miranda House College, University of Delhi, Delhi. We are thankful to Miss Lajpreet Kaur (CSIR-SRF) for her constant support and guidance.
Author information
Authors and Affiliations
Contributions
Ayushi Mishra: data curation; formal analysis; writing, original draft; writing, review and editing; conceptualization.
Jyoti Pandey: formal analysis, supervision, writing—review and editing.
Himanshu Ojha: funding acquisition, investigation, methodology, resources.
Malti Sharma: conceptualization, formal analysis.
Lajpreet Kaur: conceptualization, formal analysis, validation.
Akhilesh Pandey: Resources
Pankaj Sharma: Resources
Sumit Murab: Resources
Rahul Singhal: Resources, software
Mallika Pathak: conceptualization, formal analysis, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
This manuscript has not been published elsewhere and is not under consideration by another journal. We have approved the manuscript and agree with submission to ESPR. There are no conflicts of interest to declare.
Consent to participate and consent for publication
All the authors contributed to the research and revision of this article and mutually agree that it should be submitted to ESPR.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Magnetic Lagenaria siceraria biochar (γ-Fe2O3-LSB) was synthesized from food waste.
• γ-Fe2O3-LSB is used as an efficient adsorbent for the removal of methylene blue dye from aqueous solution.
• Adsorption isotherms, kinetic models, and thermodynamics of the adsorption process were studied.
• Cost analysis for γ-Fe2O3-LSB synthesis was calculated at a laboratory scale which comes out to be 162.75 INR/kg.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, A., Pandey, J., Ojha, H. et al. A green and economic approach to synthesize magnetic Lagenaria siceraria biochar (γ-Fe2O3-LSB) for methylene blue removal from aqueous solution. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33477-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-33477-6