Abstract
Biopesticides, having as active ingredients viruses, bacteria, or fungi, are developed to substitute or reduce the use of chemical plant protection products in different agrosystems. Though the application of mixtures containing several products is a common practice, interactions between microbial biopesticides and related effects on bees as non-target organisms have not been studied yet. In the current study, we exposed winter bees to five different microbial-based products and their combinations at the maximum recommended application rate to assess their responses. Laboratory oral exposure tests (acute/chronic) to single or binary products were conducted. Survival and food consumption of the tested bees were evaluated over the experimental duration. Our results show that some product combinations have potential additive or synergistic effects on bees, whereas others did not affect the bee’s survival compared to the control. Exposure of tested bees to the most critical combination of products containing Bacillus thuringiensis aizawai ABTS-1857 and B. amyloliquefaciens QST 713 strongly resulted in a median lifespan of 4.5 days compared to 8.0 and 8.5 days after exposure to the solo products, respectively. The exposure to inactivated microorganisms by autoclaving them did not differ from their respective uncontaminated negative controls, indicating effects on bee mortality might originate in the treatment with the different microorganisms or their metabolites. Further investigations should be conducted under field conditions to prove the magnitude of observed effects on bee colonies and other bee species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent reports have indicated increasing concerns regarding residue accumulation and potential adverse effects on the environment by chemical plant protection products (PPPs). Bees are exposed to residues of numerous active substances in various landscapes during their foraging activities (Böhme et al. 2018; Friedle et al. 2021; Mair and Wolf 2023). Among different stressors, exposure to pesticides at different concentrations in bee collected matrices, i.e. pollen and nectar, can cause complex responses at cellular mechanisms of the individuum, the whole organism, and colony/population level (e.g., Alkassab and Kirchner 2017; Siviter et al. 2021).
Nowadays, there is increasing interest in using sustainable biopesticides to reduce reliance on chemicals. Microbial biopesticides formulated from live microorganisms, including bacteria, fungi, and viruses, provide control options for several agricultural pests and diseases and fit well in integrated pest management (IPM) strategies (Quarles 2013). Such agents have been reported to have higher target-specificity than many synthetic chemicals (Mishra et al. 2020). However, their efficacy may be affected by various abiotic and biotic factors (Ownley et al. 1992). Various approaches have been suggested to overcome the limitations of efficacy, including the application of several products simultaneously by tank mixing to maximize efficacy and control a broad spectrum of pests in parallel (Bremmer et al. 2021; Haggag and Nofal 2006) and to overcome inconsistent performance under varying environmental conditions (Glare et al. 2012; Raymond et al. 2013; Van Lenteren 2012). Meshram et al. (2022) reported several ways of combinations, such as combining various microbes, combining different modes of action, and developing strain mixtures.
The simultaneous exposure, e.g., by tank mixing, to multiple microorganisms can lead to unpredictable effects on target or non-target organisms due to complex interactions. Some studies reported synergistic or antagonistic effects on target pests after combining microbial biopesticides (Garbutt et al. 2011; Hodgson et al. 2004; Li et al. 2021; Raymond et al. 2008). Soth et al. (2022) suggested that combining several genetically distinct isolates might lead to synergistic interactions or overcome environmental constraints. Studies using combinations of the two most applied entomopathogens, Bacillus thuringiensis and Beauveria bassiana, on target pests showed various interactions with different effects, including synergistic (Wraight and Ramos 2005; Kryukov et al. 2009; Xue et al. 2018), additive (Mwamburi et al. 2009), or antagonistic (Ma et al. 2008) depending on the pest and doses (Gao et al. 2012; Mantzoukas et al. 2013; Sayed and Behle 2017; Wraight and Ramos 2017). Nevertheless, combinations of microorganisms often provide more effective disease control than the single one (de Boer et al. 2003).
The use of biopesticides is expected to increase worldwide in the following years (Fenibo et al. 2021), and the simultaneous exposure of non-target organisms to multiple microorganisms should be considered and evaluated. Several guidelines have been developed to deal with the tank mixing of synthetic pesticides (Gandini et al. 2020), whereas similar guidelines are lacking for biopesticides. Some products are proven for compatibility with other products, and the label provides such information. However, this is not the case for the majority of microbial biopesticides.
Honey bees (Apis mellifera), as well as other pollinating insects, will be exposed to applied biopesticides by collecting, consuming, and storing contaminated pollen and nectar. In particular, honey bees are reported to perform hygienic behavior and grooming to minimize the risk of infections by fungi or bacteria (Facchini et al. 2019; Qu and Wang 2018). With respect to microbial biopesticides, Peng et al. (2020) reported that the average temperature of 33–36 °C within the colony during the season might work as a natural protection against fungal infections through fungal heat-inactivation. However, this cannot be generalized to all possible diseases caused by a fungal infection. Examples are fungal agents causing chalkbrood (Ascosphaera apis) and stonebrood (Aspergillus flavus, A. fumigatus, A. niger) in honey bee colonies. Despite an optimal temperature range of 30–35 °C for brood rearing, it is possible for A. apis to infect honey bee brood cells and cause chalkbrood (Flores et al. 1996).
Especially overwintering is a critical phase of colony development, where the colony forms a thermoregulating cluster to maintain the hive temperature between 24 and 34 °C during periods of cold temperatures (Heinrich 1981). Winter bees have a more extended survival capacity than summer bees. It is reported that winter bees have different physiology, e.g., a higher titer of yolk protein Vitellogenin, an enlarged fat body, and a higher number of hemocytes, which may lead to different sensitivity to infections by various microorganisms (Aurori et al. 2014; Remolina and Hughes 2008). While most studies analyzing microbial pesticides’ effects on honey bees work with summer bees (e.g., Malone et al. 1999; Renzi et al. 2016; Steinigeweg et al. 2021), the effects on winter bees are less investigated. To this end, the objective of the present study is to evaluate the effects of exposure to a tank mixture of several microbial biopesticides on long-living winter honey bees under laboratory conditions.
Materials and methods
Bee samples and experimental conditions
Winter worker honey bees (Apis mellifera) were collected from two healthy colonies maintained at the apiary of the Institute for Bee Protection of the Julius Kühn Institute in Braunschweig, Germany. All colonies had low levels of Varroa infections, where the natural mite falls lower than one per day. The treatment with formic acid against Varroa was conducted during summer 10 weeks before starting the experiment. Each colony had about 9000 workers and a fertile 1-year-old sister queen. Winter worker bees with undefined age were collected, shortly immobilized on ice and caged in groups of 10 bees. Each treatment group had four cages. The bees were incubated under controlled conditions (RH 65 ± 2%, 26 ± 2 °C, and darkness). For further details regarding cage description, see Alkassab and Kirchner (2016).
Microbial biopesticides
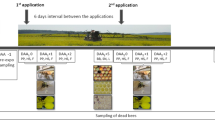
Five microbial-based products, being commercially available in Germany, and their combinations at the maximum recommended application rate were tested (Table 1). Naturalis® contains 2.3 × 107 CFU/ml of Beauveria bassiana (ATCC 74040), due to the available information on the product label. FlorBac® contains maximum 6 × 1013 CFU/kg of Bacillus thuringiensis spp. aizawai (ABTS-1857) (EFSA (European Food Safety Authority) 2020). Lepinox® Plus contains maximum 1.1 × 1013 CFU/kg Bacillus thuringiensis ssp. kurstaki (EG-2348) (EFSA (European Food Safety Authority) 2021a). Serenade® ASO contains maximum 3 × 1013 CFU/kg of Bacillus amyloliquefaciens (QST 713) (EFSA (European Food Safety Authority) 2021b). Madex® MAX contains nominal content of 3 × 1013 CpGV-M OB/L (EFSA (European Food Safety Authority) 2022). Biopesticides were prepared in the feeding solution (2 M sucrose w/v) at the manufacturer’s recommended concentrations. For combined treatments, both products were mixed at their individual recommended concentrations. The available information for compatibility with other products provided on the label was considered to select the combinations and mixing of different products.
Exposure protocols
Winter honey bees were exposed orally either acutely for 6–8 h (Organisation for Economic Co-operation and Development (OECD) 1998) or chronically over 10 days (Organisation for Economic Co-operation and Development (OECD) 2017) to the maximum recommended application rate of a single product or in a tank mixture of two microbial PPPs (Table 1). In the acute experiment, treated bees were starved for 90 min before providing 200 µl/cage of contaminated sucrose solution and were observed for 6–8 h. When this amount was consumed completely, the bees were fed ad libitum with sucrose solution (2 M) for the subsequent period. In the chronic experiment, treated bees were fed ad libitum with contaminated sucrose solution until day 10. For the subsequent 5 days, bees were fed with an uncontaminated sucrose solution (2 M). The control groups were fed ad libitum with an uncontaminated 2 M sucrose solution.
To evaluate the effect of adjuvants and co-formulants in the several products, an additional treatment group was exposed to inactivated microorganisms by autoclaving a stock solution (1% w/w) of the products solved in sterile water at 121 °C for 20 min. The Bacillus thuringiensis (B.t.)-based products were autoclaved twice to ensure the inactivation of B.t. spores. Thereafter, the autoclaved solutions were added at the same concentrations like the inactivated ones in sugar solution to evaluate their effects on bees. All test solutions were freshly prepared daily and fed to bees without storage to ensure high viability of all tested microorganisms. Mortality and food uptake were monitored daily for a total period of 15 days.
Statistical analyses
To compare the different survival rates among groups over the test duration, Kaplan–Meier tests from the R package survminer were used (Kassambara et al. 2021). Bonferroni correction was used for multiple comparisons. Cox proportional hazard models from the R package survival (Therneau et al. 2020) were used to estimate hazard ratios (HR) for solo products and tank mixtures. The synergy index (SIAB) was calculated to determine the potential interaction between the tested combinations, applying the following formula designated by de Mutsert et al. (2009): SIAB = (HRAB − 1) / (HRA + HRB − 2). If the index was > 1, this is an indication of a synergistic effect, whereas an index SI = 1 or lower indicates no interaction effect. Linear mixed effects models (LMMs) were used to account for repeated measures and to test for differences in sucrose solution consumed per bee. Models were run with the function lmer from the nlme package (Pinheiro et al. 2021). All statistical analyses were conducted using R version 4.0.3 (R Core Team 2020) at a significance level of 0.05. Graphs were created using the ggplot function from the library ggplot2 (Wickham et al. 2020).
Results
Chronic exposure
Comparing all effects of the different tank mixtures on honey bees, the results showed a strong variance in the observed effects. In the majority of tested mixtures, survival effects were driven mainly by the effects of the solo products (Naturalis® or FlorBac®), which caused the strongest effect (p < 0.001; Fig. 1A–C). Some mixtures indicated potential additive (Fig. 1D) or synergistic effects (Fig. 1E). Mixture of products containing C. pomonella GV0006 and B.t.k. EG-2348 did not affect the bee’s survival (p > 0.05; Fig. 1F).
Survival rates of adult winter honey bees over 15 days after chronic exposure for 10 days (dashed lines) to different biopesticides (solo and mix), compared to autoclaved ones and the untreated control. A Naturalis®, FlorBac®, and their mixture; B Naturalis ®, Madex® MAX and their mixture; C Naturalis®, Lepinox® Plus, and their mixture; D Madex® MAX, Serenade® ASO and their mixture; E FlorBac®, Serenade® ASO and their mixture; F Madex® MAX, Lepinox® Plus, and their mixture. (N = 4 cages/treatment, n = 10 bees/cage; Kaplan–Meier tests; asterisk indicates p < 0.05 compared to control)
The combination of products containing C. pomonella GV0006 and B.a. QST 713 caused significantly faster mortality by archiving the 50% mortality 36 h earlier than the solo product (p = 0.001; Fig. 1D). The median lifespan of the tested bees was 7.0 days after exposure to the mixture, compared to 8.5 and > 15 days after exposure to the products containing B.a. QST 713 or C. pomonella GV0006, respectively.
The critical mixture of products containing B.t.a. ABTS-1857 and B.a. QST 713 strongly reduced the survival time compared to the solo products (p < 0.001; Fig. 1E). The median lifespan of the tested bees was 4.5 days after exposure to the mixture, compared to 8.0 and 8.5 days after exposure to the products containing B.t.a. ABTS-1857 and B.a. QST 713, respectively. The autoclaved groups did not differ from their respective uncontaminated negative controls (p > 0.05; Fig. 1A–F).
Acute exposure
In general, acute exposure resulted in lower levels of mortality compared to chronic exposure to both solo products as well as their combinations (Fig. 1). The acute exposure to the mixture of products containing B.t.a. ABTS-1857 and B.b. ATCC 74040 caused an effect comparable to B.t.a. ABTS-1857 solo application (p < 0.001; Fig. 2A). Acute exposure to the mixture of products containing B.t.a. ABTS-1857 and B.a. QST 713 reduced the survival time significantly compared to the single products, which confirmed the results after chronic exposure but with lower mortality levels (p < 0.001; Fig. 2C). On the other hand, acute exposure to the mixture of products containing B.a. QST 713 and C. pomonella GV0006 did not affect the survival of tested bees (p > 0.05; Fig. 2B).
Survival rates of adult winter worker honey bees over 16 days after acute exposure for 6–8 h (dashed lines) to different biopesticides (solo and mix), compared to the untreated controls. A Naturalis®, FlorBac®, and their mixture; B Madex® MAX, Serenade® ASO and their mixture; C FlorBac®, Serenade® ASO and their mixture. (N = 4 cages/treatment, n = 10 bees/cage; Kaplan–Meier test; asterisk indicates p < 0.05 compared to control)
Effects of tested combinations
Estimated hazard ratios (HR) show the probability of a living treated bee at a certain time point that will be dead by the next time point compared to an untreated bee (Fig. S1). These values were used to calculate the synergy index for each tank mixture. Two tank mixtures show potential synergism after chronic exposure (Table 2).
The synergy indices were clearly above one for the tank mixture of the products containing B.t.a. ABTS-1857 and B.a. QST 713 after both acute (SI = 3.27) and chronic exposure (SI = 7.50), indicating a general synergism for this combination. The tank mixture of the products containing B.a. QST 713 and C. pomonella GV0006 showed potential synergism after chronic exposure (SI = 2.16) but not after acute exposure (SI = 0.00).
Food consumption
Significantly lower consumption was observed during the chronic exposure phase to Serenade® ASO (26.8 ± 4.0 µl/bee) or tank mixtures containing this product, i.e., Serenade® ASO + Madex® MAX (33.6 ± 5.0 µl/bee) and Serenade® ASO + FlorBac® (27.6 ± 6.3 µl/bee), relative to the control (46.1 ± 5.7 µl/bee) (p < 0.05; Fig. 3D, E). Treated bees with tank mixture Lepinox® Plus + Madex® MAX consumed a significantly lower amount during exposure (33.7 ± 2.6 µl/bee) and post-exposure phase (37.6 ± 2.9 µl/bee) compared to control (46.1 ± 5.7 µl/bee in the first phase and 54.5 ± 2.9 µl/bee in the second phase) (p < 0.05; Fig. 3F). The exposed bees to Madex® MAX showed significantly lower consumption in the post-exposure phase (33.7 ± 4.4 µl/bee) relative to the negative control (p < 0.05; Fig. 3B, F). No effect on food consumption was observed for the autoclaved mixtures, neither during exposure nor post-exposure.
Daily food consumption (µl/bee/day) of chronically tested honey bees during exposure (days 1–10) and observation (days 11–15) phases. A Naturalis®, FlorBac®, and their mixture; B Naturalis ®, Madex® MAX and their mixture; C Naturalis®, Lepinox® Plus, and their mixture; D Madex® MAX, Serenade® ASO and their mixture; E FlorBac®, Serenade® ASO and their mixture; F Madex® MAX, Lepinox® Plus, and their mixture. Consumptions are shown as boxplots with median; the edges of the box indicate the 25th and 75th percentiles. Treatments not sharing the same letters indicate significant differences (p < 0.05)
No significant differences between treatment groups were observed in food consumption after acute exposure, except for the autoclaved mix 1 and Florbac® (p > 0.05; Fig. S2).
Discussion
Typically, the risk assessment for bees is conducted for each plant protection product before authorization (Krahner et al. 2022). Most commercial microbial biopesticides contain a single strain of a specific microorganism. Currently, several studies attempt to investigate the efficacy improvement by applying a combination of multiple microbial biopesticides based on the assumption that infections by more than one pathogen can synergistically affect suppressing pest populations (Garbutt et al. 2011; Wraight and Ramos 2005). However, a few synergistic interactions among biopesticides have been reported (Xu et al. 2011; Kryukov et al. 2009). In any case, non-target organisms, including pollinator insects, will be exposed to a mixture of applied microorganisms directly through the tank mixture or indirectly through the residues, i.e., the microorganisms or their metabolites. Recently, we reported that bees can be exposed chronically through stored pollen and nectar within the hive after application of a product containing B.t.a. in the field. This long-term presence of B.t.a. might be explained by differences between in-hive conditions compared to field conditions, where no more UV effects on the spores and/or the products within the hive are expected (Alkassab et al. 2022). Therefore, the evaluation of the combined effect has to include the potential effects on bees as non-target organisms.
Research investigating the effect of microbial biopesticides on honey bee health is still developing (Borges et al. 2021; Erler et al. 2022), particularly their interaction with the gut microbiome (Steinigeweg et al. 2023). In the present study, we reported for the first time how microbial combinations can affect honey bee survival under laboratory conditions. By observing no increased mortality for the autoclaved groups, it became apparent that the effects on bee mortality might originate in the treatment with the different microorganisms or their metabolites.
Our results show that a mixture of products containing C. pomonella granulovirus and B. thuringiensis ssp. kustaki did not affect the bee’s survival. This may be related to the high level of specificity of these microbials (Erler et al. 2022). However, slightly lower consumption during exposure phase was observed compared to the control. This may be related to different substances used in the formulated products. The observed effects of the other tested mixtures were driven mainly by the effects of the solo products, which caused the strongest effects. A critical mixture of products containing B. thuringiensis ssp. aizawai and B. amyloliqueaciens was identified, as the hazard ratio increased significantly compared with the solo products after acute and chronic exposure (Fig. S1).
The most studied combinations of microorganisms against target pests are B.t. and B.b. Lewis et al. (1996) reported enhanced suppression of the European corn borer after applying B.t. to B.b.-treated maize. Another study showed that applying a combination of B.b. and B.t. tenebrionis is more effective in controlling Colorado potato beetle larvae (Wraight and Ramos 2005). These effects may be related to the different modes of action of both microorganisms. B.b. can cause infections through the penetration of the cuticle, especially during the molting phase, and B.t. infects the digestive tract (Ma et al. 2008). Our results did not show such an increasing impact on adult winter honey bees after exposure to both microorganisms, as the tested bees were imagos, and thus, further investigations are still relevant to assess the effect of such combinations on bee larvae.
A strong combined effect of B.t. and B. subtilis was reported by Rajamanickam et al. (2015). They found that the lethal concentration values for the moth Helicoverpa armigera were significantly lower in the mixture than in B.t. and B.s. individually. This is in line with the results of this study, where the mixture reduced the lethal time compared to the solo products (Fig. 1). Although, B. amyloliqueaciens (formerly subtilis) is known to be effective against fungal plant diseases and produce several antibiotics as well as metabolites; among them, the metabolites surfactin and iturin have been reported to have insecticidal and insect antifeedant activity (Assié et al. 2002; Geetha and Manonmani 2010; Blibech et al. 2012). In the honey bee, the natural occurrence of several strains of B. subtilis was reported in the bee’s gut (Sabaté et al. 2009). On the other hand, ingestion of extrinsic strains of B. subtilis or its metabolites was shown to affect other insects adversely (Abd El-Salam et al. 2011; Ghribi et al. 2012). B. thuringiensis ssp. aizawai is an effective entomopathogen against lepidopteran larvae. However, its semi-specificity was indicated recently depending on the reported cross-effects across insect taxa and orders (e.g., Babin et al. 2020; Nawrot-Esposito et al. 2020; Steinigeweg et al. 2021, 2023; Tudoran et al. 2020; van Frankenhuyzen 2017). This shows that target-specificity is not always as strong as expected. Exposure to a mixture of spores with a different spectrum of metabolites from both bacterial species can increase the stress level within the digestive system. Potential digestive problems may be the result of a disruption in the microbiome’s function or of foreign microorganisms causing dysbiosis in the gut microbiome (Erler et al. 2022). A digestive problem may be related to the lower consumption rate during exposure to this mixture. Thus, more omics research can provide more information on the host’s physiology and metabolism related to the observed combined effects. Furthermore, the host species and inoculation strategy can affect the prevalence and consequences of co-infection situations. Studies investigating the interaction between microbials and their target and non-target host organisms will lead to a better understanding of their effects (Souza et al. 2019; Erler et al. 2022).
Conclusion
The current study assessed separately the effects of five microbial biopesticides and some combinations to determine if there is a mixture with a critical impact on bees. Survival reductions were observed after chronic exposure to solo products containing B.t.a. ABTS-1857, B.a. QST 713, and B.b. ATCC 74040, whereas no effects of the products containing C. pomonella GV0006 and B. t. k. EG-2348 were detectable. Chronic exposure resulted in higher levels of mortality compared with acute exposure to solo products. This shows that combined exposure to microbial plant protection products has the potential, in some cases, to increase the risk for non-target organisms like honey bees. In such cases, further higher-tier studies with full-size colonies are needed to assess the response of honey bees and the related risk for honey bee colonies under field conditions.
Supplementary information.
Data availability
Data will be made available on reasonable request.
References
Abd El-Salam AME, Nemat AM, Magdy A (2011) Potency of Bacillus thuringiensis and Bacillus subtilis against the cotton leafworm, Spodoptera littoralis (Bosid.) larvae. Arch Phytopathol Plant Prot 44:204–215
Alkassab AT, Kirchner WH (2016) Impacts of chronic sublethal exposure to clothianidin on winter honeybees. Ecotoxicology 25:1000–1010
Alkassab AT, Beims H, Janke M, Pistorius J (2022) Determination, distribution, and environmental fate of Bacillus thuringiensis spores in various honeybee matrices after field application as plant protection product. Environ Sci Pollut Res 29:25995–26001
Alkassab AT, Kirchner WH (2017) Sublethal exposure to neonicotinoids and related side effects on insect pollinators: honeybees, bumblebees, and solitary bees. J Plant Dis Prot 124:1–30
Assié LK, Deleu M, Arnaud L, Paquot M, Thonart P, Ch G, Haubruge E (2002) Insecticide activity of surfactins and iturins from a biopesticide Bacillus subtilis Cohn (S499 strain). Mededelingen (Rijksuniversiteit te Gent. Fakulteit van de Landbouwkundige en Toegepaste Biologische Wetenschappen) 67 647–655
Aurori CM, Buttstedt A, Dezmirean DS, Mărghitaş LA, Moritz RF, Erler S (2014) What is the main driver of ageing in long-lived winter honeybees: antioxidant enzymes, innate immunity, or vitellogenin? J Gerontol A Biomed Sci Med Sci 69:633–639
Babin A, Nawrot-Esposito MP, Gallet A, Gatti JL, Poirié M (2020) Differential side-effects of Bacillus thuringiensis bioinsecticide on non-target Drosophila flies. Sci Rep 10:1–16
Behrends A, Scheiner R (2010) Learning at old age: a study on winter bees. Front Behav Neurosci 4:1563
Blibech I, Ksantini M, Chaieb I, Jlassi B, Rhouma A, Jaoua S, Aifa S (2012) Isolation of entomopathogenic Bacillus from a biodynamic olive farm and their pathogenicity to lepidopteran and coleopteran insect pests. Crop Prot 31:72–77
Böhme F, Bischoff G, Zebitz CP, Rosenkranz P, Wallner K (2018) Pesticide residue survey of pollen loads collected by honeybees (Apis mellifera) in daily intervals at three agricultural sites in South Germany. PLoS ONE 13:e0199995
Borges S, Alkassab AT, Collison E, Hinarejos S, Jones B, McVey E et al (2021) Overview of the testing and assessment of effects of microbial pesticides on bees: strengths, challenges and perspectives. Apidologie 52:1256–1277
Bremmer J, Riemens M, Reinder M. (2021) The future of crop protection in Europe, 1st ed.; Scientific Foresight Unit (STOA): Brussels, Belgium. https://www.europarl.europa.eu/RegData/etudes/STUD/2021/656330/EPRS_STU(2021)656330_EN.pdf
de Boer M, Bom P, Kindt F, Keurentjes JJ, van der Sluis I, Van Loon LC, Bakker PA (2003) Control of Fusarium wilt of radish by combining Pseudomonas putida strains that have different disease-suppressive mechanisms. Phytopathology 93:626–632
de Mutsert R, Jager KJ, Zoccali C, Dekker FW (2009) The effect of joint exposures: examining the presence of interaction. Kidney Int 75:677–681
EFSA (European Food Safety Authority) (2020) Conclusion on the peer review of the pesticide risk assessment of the active substance Bacillus thuringiensis ssp aizawai strain ABTS-1857. EFSA J 18(10):6294
EFSA (European Food Safety Authority) (2021a) Conclusion on the peer review of the pesticide risk assessment of the active substance Bacillus amyloliquefaciens strain QST 713 (formerly Bacillus subtilis strain QST 713). EFSA J 19(1):6381
EFSA (European Food Safety Authority) (2021b) Conclusion on the peer review of the pesticide risk assessment of the active substance Bacillus thuringiensis subsp. kurstaki strain EG 2348. EFSA J 19(4):6495
EFSA (European Food Safety Authority) (2022) Conclusion on the peer review of the pesticide risk assessment of the active substance Cydia pomonella granulovirus (CpGV). EFSA J 20(11):7630
Erler S, Eckert JH, Steinert M, Alkassab AT (2022) Impact of microorganisms and entomopathogenic nematodes used for plant protection on solitary and social bee pollinators: host range, specificity, pathogenicity, toxicity, and effects of experimental parameters. Environ Pollut 302:119051
Facchini E, Bijma P, Pagnacco G, Rizzi R, Brascamp EW (2019) Hygienic behaviour in honeybees: a comparison of two recording methods and estimation of genetic parameters. Apidologie 50:163–172
Fenibo EO, Ijoma GN, Matambo T (2021) Biopesticides in sustainable agriculture: a critical sustainable development driver governed by green chemistry principles. Front Sustain Food Syst 5:619058
Flores JM, Ruiz JA, Ruz JM, Puerta F, Bustos M, Padilla F, Campano F (1996) Effect of temperature and humidity of sealed brood on chalkbrood development under controlled conditions. Apidologie 27:185–192
Friedle C, Wallner K, Rosenkranz P, Martens D, Vetter W (2021) Pesticide residues in daily bee pollen samples (April–July) from an intensive agricultural region in Southern Germany. Environ Sci Pollut Res 28:22789–22803
Gandini EM, Costa ES, dos Santos JB, Soares MA, Barroso GM, Corrêa JM, Carvalho AG, Zanuncio JC. (2020) Compatibility of pesticides and/or fertilizers in tank mixtures. J Clean Prod 268:122152
Gao Y, Oppert B, Lord JC, Liu C, Lei Z (2012) Bacillus thuringiensis Cry3Aa toxin increases the susceptibility of Crioceris quatuordecimpunctata to Beauveria bassiana infection. J Invertebr Pathol 109:260–263
Garbutt J, Bonsall MB, Wright DJ, Raymond B (2011) Antagonistic competition moderates virulence in Bacillus thuringiensis. Ecol Lett 14:765–772
Geetha I, Manonmani AM (2010) Surfactin: a novel mosquitocidal biosurfactant produced by Bacillus subtilis ssp. subtilis (VCRC B471) and influence of abiotic factors on its pupicidal efficacy. Lett Appl Microbiol 51:406–412
Ghribi D, Abdelkefi-Mesrati L, Mnif I, Kammoun R, Ayadi I, Saadaoui I et al (2012) Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J Biomed Biotechnol 2012:373682
Glare T, Caradus J, Gelernter W, Jackson T, Keyhani N, Köhl J et al (2012) Have biopesticides come of age? Trends Biotechnol 30:250–258
Haggag WM, Nofal MA (2006) Improving the biological control of Botryodiplodia disease on some Annona cultivars using single or multi-bioagents in Egypt. Biol Control 38:341–349
Heinrich B (1981) The mechanisms and energetics of honeybee swarm temperature regulation. J Exp Biol 91:25–55
Hodgson DJ, Hitchman RB, Vanbergen AJ, Hails RS, Possee RD, Cory JS (2004) Host ecology determines the relative fitness of virus genotypes in mixed-genotype nucleopolyhedrovirus infections. J Evol Biol 17:1018–1025
Kassambara A, Kosinski M, Biecek P, Fabian S (2021) survminer: Drawing Survival Curves using’ggplot2’. R package version 0.4. 9. 2021
Krahner A, Alkassab AT, Jütte T, Lüken DJ, Wirtz IP, Pistorius J (2022) Review of approaches to the pesticide regulatory risk assessment for bees and other pollinators. (Berichte aus dem Julius Kühn-Institut 219). Braunschweig, Deutschland, 50 Seiten. Doi: https://doi.org/10.5073/20220128-114629
Kryukov VY, Khodyrev VP, Yaroslavtseva ON, Kamenova AS, Duisembekov BA, Glupov VV (2009) Synergistic action of entomopathogenic hyphomycetes and the bacteria Bacillus thuringiensis ssp. morrisoni in the infection of Colorado potato beetle Leptinotarsa decemlineata. Appl Biochem Microbiol 45:511–516
Lewis LC, Berry EC, Obrycki JJ, Bing LA (1996) Aptness of insecticides (Bacillus thuringiensis and carbofuran) with endophytic Beauveria bassiana, in suppressing larval populations of the European corn borer. Agr Ecosyst Environ 57:27–34
Li S, Yi W, Chen S, Wang C (2021) Empirical support for the pattern of competitive exclusion between insect parasitic fungi. J Fungi 7:385
Ma XM, Liu XX, Ning X, Zhang B, Han F, Guan XM et al (2008) Effects of Bacillus thuringiensis toxin Cry1Ac and Beauveria bassiana on Asiatic corn borer (Lepidoptera: Crambidae). J Invertebr Pathol 99:123–128
Mair B, Wolf M (2023) Studies on the botanical origin and the residues of pesticides in corbicular pollen loads and bee bread of bee colonies in the proximity of apple orchards in South Tyrol. J Cultivated Plants 75:225–234
Malone LA, Burgess EPJ, Stefanovic D (1999) Effects of a Bacillus thuringiensis toxin, two Bacillus thuringiensis biopesticide formulations, and a soybean trypsin inhibitor on honey bee (Apis mellifera L.) survival and food consumption. Apidologie 30:465–473
Mantzoukas S, Milonas P, Kontodimas D, Angelopoulos K (2013) Interaction between the entomopathogenic bacterium Bacillus thuringiensis subsp: Kurstaki and two entomopathogenic fungi in bio-control of Sesamia nonagrioides (Lefebvre) (Lepidoptera: Noctuidae). Ann Microbiol 63:1083–1091
Meshram S, Bisht S, Gogoi R (2022) Current development, application and constraints of biopesticides in plant disease management. In Biopesticides (pp. 207–224) Woodhead Publishing
Mishra J, Dutta V, Arora NK (2020) Biopesticides in India: technology and sustainability linkages. 3 10:210
Mwamburi LA, Laing MD, Miller R (2009) Interaction between Beauveria bassiana and Bacillus thuringiensis var. israelensis for the control of house fly larvae and adults in poultry houses. Poult Sci 88:2307–2314
Nawrot-Esposito MP, Babin A, Pasco M, Poirié M, Gatti JL, Gallet A (2020) Bacillus thuringiensis bioinsecticides induce developmental defects in non-target Drosophila melanogaster larvae. Insects 11:697
Organisation for Economic Co-operation and Development (OECD) (1998) OECD guidelines for the testing of chemicals honeybees, acute oral toxicity test 213/ Adopted Sep. 21, 1998. https://www.oecd-ilibrary.org/environment/test-no-213-honeybees-acute-oral-toxicity-test_9789264070165-en
Organisation for Economic Co-operation and Development (OECD) (2017) OECD guideline for the testing of chemicals. Honey bee (Apis mellifera), chronic oral toxicity test (10-day feeding). OECD/OCDE 245. Adopted Oct. 9, 2017. https://www.oecd-ilibrary.org/docserver/9789264284081-en.pdf?expires=1586028842&id=id&accname=guest&checksum=F78F26502CCEE0CBABA58FF0475031F4
Ownley BH, Weller DM, Thomashow LS (1992) Influence of in situ and in vitro pH on suppression of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens 2–79. Phytopathology 82:178–184
Peng G, Tong SM, Zeng D, Xia Y, Feng MG (2020) Colony heating protects honey bee populations from a risk of contact with wide-spectrum Beauveria bassiana insecticides applied in the field. Pest Manag Sci 76:2627–2634
Pinheiro J, Bates, D, DebRoy S, Sarkar D (2021) R. Core Team. 2021. nlme: linear and nonlinear mixed effects models. R package version 3.1‐152. J Apic Res
Qu S, Wang S (2018) Interaction of entomopathogenic fungi with the host immune system. Dev Comp Immunol 83:96–103
Quarles W (2013) New biopesticides for IPM and organic production. IPM Pract 33:1–20
R Core Team (2020) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing
Rajamanickam C, Kannan R, Somannan J (2015) Combined effect of Bacillus thuringiensis and Bacillus subtilis against Helicoverpa armigera. Int J Curr Microbiol App Sci 4:127–141
Raymond B, Lijek RS, Griffiths RI, Bonsall MB (2008) Ecological consequences of ingestion of Bacillus cereus on Bacillus thuringiensis infections and on the gut flora of a lepidopteran host. J Invertebr Pathol 99:103–111
Raymond B, Wright DJ, Crickmore N, Bonsall MB (2013) The impact of strain diversity and mixed infections on the evolution of resistance to Bacillus thuringiensis. Proc R Soc B Biol Sci 280:20131497
Remolina SC, Hughes KA (2008) Evolution and mechanisms of long life and high fertility in queen honey bees. Age 30:177–185
Renzi MT, Amichot M, Pauron D, Tchamitchian S, Brunet JL, Kretzschmar A, Maini S, Belzunces LP (2016) Chronic toxicity and physiological changes induced in the honey bee by the exposure to fipronil and Bacillus thuringiensis spores alone or combined. Ecotoxicol Environ Saf 127:205–213
Sabaté DC, Carrillo L, Audisio MC (2009) Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res Microbiol 160:193–199
Sayed AM, Behle RW (2017) Comparing formulations for a mixed-microbial biopesticide with Bacillus thuringiensis var. kurstaki and Beauveria bassiana blastospores. Arch Phytopathol Plant Prot 50:745–760
Siviter H, Richman SK, Muth F (2021) Field-realistic neonicotinoid exposure has sub-lethal effects on non-Apis bees: a meta-analysis. Ecol Lett 24:2586–2597
Soth S, Glare TR, Hampton JG, Card SD, Brookes JJ (2022) Biological control of diamondback moth—increased efficacy with mixtures of Beauveria fungi. Microorganisms 10:646
Souza ML, Sanches MM, de Souza DA, Faria M, Espinel-Correal C, Sihler W, Lopes RB (2019) Within-host interactions of Metarhizium rileyi strains and nucleopolyhedroviruses in Spodoptera frugiperda and Anticarsia gemmatalis (Lepidoptera: Noctuidae). J Invertebr Pathol 162:10–18
Steinigeweg C, Alkassab AT, Beims H, Eckert JH, Richter D, Pistorius J (2021) Assessment of the impacts of microbial plant protection products containing Bacillus thuringiensis on the survival of adults and larvae of the honeybee (Apis mellifera). Environ Sci Pollut Res 28:29773–29780
Steinigeweg C, Alkassab AT, Erler S, Beims H, Wirtz IP, Richter D, Pistorius J (2023) Impact of a microbial pest control product containing Bacillus thuringiensis on brood development and gut microbiota of Apis mellifera worker honey bees. Microb Ecol 85:1300–1307
Therneau T, Crowson C, Atkinson E (2020) Multi-state models and competing risks. CRAN-R (https://cran.r-project.org/web/packages/survival/vignettes/compete.pdf.
Tudoran A, Nordlander G, Karlberg A, Puentes A (2020) A major forest insect pest, the pine weevil Hylobius abietis, is more susceptible to Diptera-than Coleoptera-targeted Bacillus thuringiensis strains. Pest Manag Sci 77:1303–1315
van Frankenhuyzen K (2017) Specificity and cross-order activity of Bacillus thuringiensis pesticidal proteins. In: Bacillus thuringiensis and Lysinibacillus sphaericus. Springer Cham, pp 127–172
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20
Wickham H, François R, Henry L, Müller K (2020). Dplyr: a Grammar of Data Manipulation. R package version 0.8.5. Retrieved Oct. 19, 2020, from. https://CRAN.R-project.org/package=dplyr
Wraight SP, Ramos ME (2005) Synergistic interaction between Beauveria bassiana and Bacillus thuringiensis tenebrionis-based biopesticides applied against field populations of Colorado potato beetle larvae. J Invertebr Pathol 90:139–150
Wraight SP, Ramos ME (2017) Characterization of the synergistic interaction between Beauveria bassiana strain GHA and Bacillus thuringiensis morrisoni strain tenebrionis applied against Colorado potato beetle larvae. J Invertebr Pathol 144:47–57
Xu D, Ali S, Huang Z (2011) Insecticidal activity influence of 20-Hydroxyecdysone on the pathogenicity of Isaria fumosorosea against Plutella xylostella. Biol Control 56:239–244
Xue C, Sun Y, Zhang Y (2018) Influence of synergism with Bacillus thuringiensis and Beauveria bassiana on diamondback moth larvae. In Proceedings of the 7th International Conference on Energy, Environment and Sustainable Development (ICEESD 2018), Shenzhen, China, 30–31 March 2018; pp. 340–343.
Acknowledgements
We would like to thank the technicians at the Institute for Bee Protection (JKI) for their assistance, as well as Tina Feer (TU Braunschweig) for her support in carrying out the experiments and data collection.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by funds of the German Federal Ministry of Food and Agriculture (BMEL).
Author information
Authors and Affiliations
Contributions
ATA experimental design, performed the statistical analyses, writing the first draft. SE experimental design, writing, and editing. MS and JP supervision, writing, and editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Giovanni Benelli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alkassab, A.T., Erler, S., Steinert, M. et al. Exposure of honey bees to mixtures of microbial biopesticides and their effects on bee survival under laboratory conditions. Environ Sci Pollut Res 31, 26618–26627 (2024). https://doi.org/10.1007/s11356-024-32753-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32753-9