Abstract
Herein, we explore the holistic integration of magnetite-based photocatalysts and techno-economic analysis (TEA) as a sustainable approach in wastewater treatment aligned with the Sustainable Development Goals (SDGs). While considerable attention has been devoted to photocatalytic dye degradation, the nexus between these processes and techno-economic considerations remains relatively unexplored. The review comprehensively examines the fundamental characteristics of magnetite-based photocatalysts, encompassing synthesis methods, composition, and unique properties. It investigates their efficacy in photocatalytic degradation, addressing homogeneous and heterogeneous aspects while discussing strategies to optimize photodegradation efficiency, including curbing electron–hole recombination and mitigating scavenging effects and interference by ions and humic acid. Moreover, the management aspects of magnetite-based photocatalysts are examined, focusing on their reusability and regeneration post-dye removal, along with the potential for reusing treated wastewater in relevant industrial applications. From a techno-economic perspective, the study evaluates the financial feasibility of deploying magnetite-based photocatalysts in wastewater treatment, correlating reduced pollution and the marketing of treated water with social, economic, and environmental objectives. By advocating the integration of magnetite-based photocatalysts and TEA, this paper contributes insights into scalable and profitable sustainable wastewater treatment practices. It underscores the alignment of these practices with SDGs, emphasizing a comprehensive and holistic approach to managing wastewater in ways that meet environmental, economic, and societal objectives.
Graphical Abstract

Similar content being viewed by others
Introduction
Dyes serve as essential components in a wide array of commercial industries, including textiles, clothing, cosmetics, food, beverages, and pharmaceuticals. Regrettably, the textile manufacturing sector alone releases over 200,000 tonnes of dyes annually into aquatic ecosystems, triggering grave environmental concerns (Abdelhaleem et al. 2022). The consequences of dye-related pollution extend beyond environmental disturbances. Human health is risked through direct skin contact or oral ingestion, as dye residues may accumulate in biological tissues and cells. The introduction of dyes into aquatic systems can engender multiple issues, spanning from altered water transparency to the emergence of toxicity, carcinogenicity, and mutagenicity (Kiron 2021a). Such pollution takes a toll on surface water ecosystems, impairing the photosynthetic capabilities of aquatic plants due to diminished sunlight penetration (Lellis et al. 2019). The implications are not confined to aquatic environments; the chemical residues from textile industrial effluents can disrupt the nutrient balance in marine ecosystems, affecting the entire human food chain, especially fish and seafood products (Hussain et al. 2020). It is imperative to recognize that some dye molecules break down into complex chemical compounds and persistent pollutants, further deteriorating natural water resources, including ponds, lakes, streams, and rivers (Pipil et al. 2022). From a human health perspective, the presence of high dye concentrations can inflict harm on vital organs such as the liver, kidneys, skin, and nervous system. Given the multifaceted and far-reaching negative impacts, the urgent need arises to curtail dye contamination within industrial effluents to safeguard environmental and human well-being.
In recent years, advanced oxidation processes (AOPs) have emerged as a promising alternative for the decolorization and mineralization of dye-rich wastewater, offering several advantages over conventional techniques (Tawfik et al. 2022). Conventional methods like biological treatment, adsorption, and coagulation/flocculation often suffer from various shortfalls, such as extended reaction periods, substantial sludge generation, and incomplete decolorization (Alkaim et al. 2014). A key factor in the success of AOPs for dye removal lies in the generation of reactive oxygen species (ROS) from a primary oxidant, such as hydrogen peroxide or ozone, or through reactions involving charge carriers, like electrons (Mourdikoudis et al. 2018). The generated ROS are then able to effectively and non-selectively degrade contaminants such as dyes (Farhadian and Farhadian 2016).
Photocatalysis, a subset of AOPs, involves the activation of a semiconductor material using natural or artificial light sources, typically with energy equal to or higher than the semiconductor’s bandgap energy (Osman et al. 2023). This process is instrumental in the degradation of dyes and can be categorized into two main approaches: heterogeneous photocatalysis: this method involves the use of a nanocatalyst, such as zinc oxide (ZnO) and titanium dioxide (TiO2), in conjunction with UV light (Byrne et al. 2015), and homogeneous photocatalytic processes including Fenton and photo-Fenton methods (Moradi et al. 2021).
Recent studies have highlighted the potential of AOPs for the removal of synthetic dyes. These processes efficiently break down complex dyes into simpler organic compounds, offering cost-effective and time-efficient solutions (Ajmal et al. 2014; Bastos-Arrieta et al. 2018; Karim et al. 2022). In a study conducted by Ahmed and Haider (2018), the benefits of heterogeneous photocatalysis for textile effluent treatment were underscored, including (i) heterogeneous photocatalysis can transform dyes into CO2 and various inorganic substances; (ii) this process occurs at standard temperature and pressure, enhancing practicality; (iii) importantly, it avoids the excessive generation of sludge, reducing waste concerns; (iv) the process primarily relies on the presence of oxygen and energy from sunlight, making it sustainable and environmentally friendly; and (vi) catalysts can be supported on a range of inert matrices, including glasses, polymers, carbon nanotubes, and graphene oxides. Furthermore, the prepared catalysts can be both cost-effective and environmentally friendly, as they are often cheap, non-toxic, and reusable (Karim et al. 2022), making an environmentally friendly approach for dye removal.
Magnetite (Fe3O4) emerges as a compelling iron oxide photocatalyst with a unique set of attributes, making it a valuable choice for various applications (Osman et al. 2022). Notably, this photocatalytic material exhibits remarkable magnetic and electrical properties, a narrow band gap, exceptional thermal stability, and biocompatibility (Rajamohan et al. 2017). One of the standout features of Fe3O4 photocatalysts is their simple recyclability, which significantly curbs the generation of secondary pollutants during the treatment processes (Mishra et al. 2019). However, it is worth noting that pure Fe3O4 is susceptible to photo-corrosion when exposed to prolonged light irradiation. To counter this limitation, it is often integrated with other semiconducting materials, a strategy that aids in charge carrier conveyance and transport (Weng et al. 2019). This integration prevents the excessive accumulation of charge carriers on the photocatalyst’s surface, enhancing light absorption by shifting the valence band (VB) and conduction band (CB) positions. Given the interplay of cost and efficiency, the utilization of AOPs based on photocatalytic semiconductors in treating textile industrial effluents is a critical area of research. The degradation of dyes using magnetite-related photocatalysts is influenced by several key factors, including solution pH, temperature, pollutant concentrations, irradiation time, and photocatalyst dosage (Abdelhaleem et al. 2022; Manohar et al. 2021). To optimize the efficacy of different magnetite-based photocatalysts, it is essential to define and apply the most suitable operating conditions. These conditions play a pivotal role in achieving higher levels of dye decolorization efficiency, a goal of paramount importance in wastewater treatment processes.
The application of magnetite-based photocatalysis to treat dye-laden wastewater holds immense promise in safeguarding environmental integrity (Moradi et al. 2021). However, in the pursuit of comprehensive sustainability, it is imperative to address certain challenges that align with the economic and social dimensions, guided by the principles of the United Nations’ sustainable development goals (SDGs) (Weng et al. 2019). These 17 SDGs encompass a framework of global objectives adopted by UN member states, aiming to secure global peace and prosperity in the present and future (Hák et al. 2016). Treating effluents from the textile industry to preserve aquatic ecosystems directly correlates with SDG6, “Clean water and sanitation,” and SDG14, “Life below water.” Furthermore, safeguarding human health against the toxicity of textile dyes aligns with the targets of SDG3, “Good health and well-being.”
In addition to these direct associations, the high degradation efficiency and reusability performance of magnetite-based photocatalysts contribute to the reduction of solid waste disposal on land and open-air burning, thus aligning with SDG15, “Life on land.” Moreover, the promotion of innovation and industrialization in photocatalyst synthesis opens new avenues for the creation of environmentally friendly jobs, supporting SDG8, “Decent work and economic growth,” through employment in sustainable projects (Pandit et al. 2023). The synthesis and application of magnetite-based photocatalysts in treating textile effluents offer not only direct but also indirect interlinkages with all 17 SDGs. This interplay underscores the need for a comprehensive exploration of the potential contributions of these photocatalysts to advancing the broader agenda of sustainable development.
To the best of the authors’ knowledge, there is a research gap in addressing the sustainable strategies for dye-laden wastewater treatment. This review paper aims to bridge the gap in existing research by examining the interconnection between photocatalytic dye degradation, techno-economic considerations, photocatalyst management, and their alignment with the social, economic, and environmental dimensions of the SDGs. The primary focus is on the utilization of heterogeneous advanced oxidation processes (AOPs) facilitated by magnetite-based photocatalysts for treating textile industrial effluents, employing a bibliometric approach. The comprehensive scope encompasses factors influencing AOP efficiency, the synthesis and characterization of magnetite-based photocatalysts for dye photodegradation, the economic feasibility of commercial-scale wastewater treatment, strategies for managing treated effluent and spent photocatalysts, and the achievement of relevant SDGs through pollution reduction. The review concludes by addressing challenges, providing recommendations for future research, and underlining the significance of the findings and their potential implications.
Dyes: types and structures
Different types of dyes have been subjected to the photocatalytic degradation process (Table 1) using magnetite-based photocatalysts. This study highlights the seven types of dyes, namely, azo dyes (Benkhaya et al. 2020; Ogugbue and Sawidis 2011), acid dyes (Kumar et al. 2021; Said et al. 2020), basic dyes (dos Santos et al. 2007; Kiron 2013), vat dyes (dos Santos et al. 2007), reactive dyes (Said et al. 2020), disperse dyes (Kiron 2021b), and sulphur dyes (Chakraborty 2011; El-Sikaily et al. 2012). A detailed discussion of the characteristics of the different dye types is given in the supplementary material.
Mechanism and fundamentals of photocatalysis
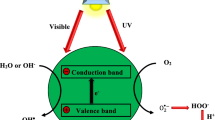
Photocatalysis obeys a series of photo-activated chemical reactions (i.e., chemical conversion using photonic energy) at a solid surface, usually a semiconductor (e.g., TiO2). A photocatalyst material is exposed to light emitting with photons that possess energy equal to or greater than the band-gap energy (Kumari et al. 2023). Subsequently, electron excitation caused an electron to transfer from the valence band (VB) to the conduction band (CB) of the photocatalyst (Eq. 1). This sequence of events generates very reactive electron–hole (e−/h+) pairs, forming complex oxidative-reductive chain reactions (Ren et al. 2021).
where e−(CB) and h+(VB) represent the electrons in the conduction band and the electron vacancy in the valence band, respectively.
Following the generation of the e−/h+ pairs, either one or two processes might occur, as shown in Fig. 1.
The first pathway is e−/h+ recombination (e− + h+ → heat), where the electron in CB surpasses the energy gap and transfers back to the valence band. The gained photon energy is dissipated, and then, the excited electrons become stable to occupy the position of the hole left behind in VB. However, the e−/h+ pair recombination should be inhibited using electron scavengers (surface adsorbents) with a larger electron storage capacity. Mixing noble metals, such as silver, gold, and platinum, with the semiconductor photocatalyst is another method to avoid the recombination between e− and h+. The second process occurs when electron donors and/or acceptors are present in the environment surrounding the semiconductor material. The electrons initially accumulate on the photocatalyst surface and commence a cascade of oxidation (oxidation of an electron donor by the holes on the semiconductor surface) and reduction (diffusion of acceptor and reduction by the electrons on the semiconductor surface) reactions (Ajmal et al. 2014). After electron excitation, a series of pollutant degradation mechanisms occurs. Three phases, i.e., (a) water ionization, (b) oxygen ionosorption, and (c) superoxide protonation, could be used to describe the degradation mechanisms (Fig. 1) (Biswas et al. 2022). Ionization of water (Eq. 2) involves the reaction of the generated valence band holes, h+(VB), with water adsorbed on the photocatalyst surface, producing hydroxyl radicals (•OH).
The generated •OH radicals are highly reactive species that react with the dyes adsorbed onto the photocatalyst surface, converting them into intermediate species (Ahmed and Haider 2018). The formed intermediate by-products are continuously subjected to this strong oxidant until they are completely broken down into final degradation products such as CO2 and H2O.
The second set of reactions represents oxygen ionosorption (Eq. 3). In this pathway, the oxygen molecules in the photocatalyst environment are combined with the CB electrons, forming a superoxide radical (O2•−). This anion of oxygen exhibits strong oxidation of dye molecules adsorbed on the photocatalyst surface continuously, breaking them down into final degradation products. The formation of O2•− radicals also assists in reducing the e−/h+ pair recombination (Ajmal et al. 2014).
The third set of reactions is termed as protonation of superoxide radicals. As such, the O2•− (superoxide anion) is protonated (reacts with the H+ ions) to form hydroperoxyl radicals (HOO•−) (Eq. 4) (Ajmal et al. 2014; Hoffmann et al. 1995). As more HOO•− are formed, they tend to react together, forming hydrogen peroxide and oxygen in the solution (Eq. 5). The H2O2 molecules then proceed to spontaneously dissociate without discernible energy barrier into more •OH (Eq. 6):
The •OH produced in the previous reactions can react with dye, causing decolouration (Eqs. 7&8):
In addition to these reactions, e− (CB) and h+ (VB) can react directly with pollutants adsorbed on the photocatalyst surface via the reduction and oxidation reactions, respectively. Furthermore, the final degradation products desorb and detach from the photocatalyst surface into the bulk solution (Ajmal et al. 2014).
Classification of magnetite-based photocatalysts
Magnetite-based photocatalysts, by definition, are photocatalysts that comprise magnetite (Fe3O4) in their chemical structure. Fe3O4-contained photocatalysts can be distinguished by their shape (e.g., spheres, rods, and flowers) or composition (composites, hybrids, or single-phase particles) (Fig. 2).
Multipurpose magnetite nanorods were synthesized using a solution-phase method in the existence of polyethylene glycol-1000 (Lian et al. 2003). The synthesized homogenous nanorods exhibited a single crystal structure, and the rod’s average diameter and length were 80 nm and 2 µm, respectively (Lian et al. 2003).
Porous Fe3O4 nanospheres were synthesized by solvothermal method with FeCl3·6H2O, polyvinylpyrrolidone (PVP), and sodium acetate as the sole iron supply, the capping agent, and the precipitation agent, respectively (Zhu and Diao 2011). The as-prepared nanospheres had an average diameter of 250 nm due to the agglomeration of smaller Fe3O4 (Zhu and Diao 2011). The nanosphere material was featured by a highly porous structure with pore sizes in the 3–10 nm range and a surface area of ≈ 47.7 m2/g. This feature was suitable for the efficient degradation of xylenol orange (XO) in an aqueous solution with H2O2 as an oxidant.
Magnetite nanoflowers were synthesized in polyol solvents using N-methyl diethanolamine as a co-solvent through a non-classical crystallization route (Gallo-Cordova et al. 2022). The nanoflower material had a particle size of about 10 nm formed from the clustering of smaller Fe3O4 particles (3–4 nm in size). These multicore nanoparticles were applied for Fenton-like photocatalytic degradation of methyl orange under visible light and displayed high degradation reaction rates up to 8.8 10−2 min−1 (Gallo-Cordova et al. 2022).
In nano-composite structures, at least one of the phases of the combined materials has a nanoscale morphology. For example, a Fe3O4@SiO2@TiO2 nanocomposite was synthesized by the coprecipitation method and used for the photocatalytic degradation of methyl orange (Mazhari & Hamadanian 2018). The nanocomposite comprised a combination of three nanosized materials, viz., a nanoscale Fe3O4 core was coated with nanosized SiO2, which was again coated with nanosized TiO2 to form Fe3O4@SiO2@TiO2. The nanocomposite displayed high surface roughness, corresponding to a specific surface area of 257.67 m2/g and an average size of ~ 25 nm.
Nanohybrid material is not only described as a type of nanocomposite material but also contains an integration of organic and inorganic phases. The combination of two or more pre-synthesized nanomaterials could adapt the nanohybrids’ physicochemical properties and introduce new properties (Aich et al. 2014). A study by Xu et al. (2022) represented the synthesis of Chlorella@Fe3O4@BiOCl (CFB) microrobots for photocatalytic degradation of rhodamine B (RhB) dye. The CFB manufacturing process included the deposition of already prepared Fe3O4 onto Chlorella cells followed by the attachment of BiOCl nanosheets to the Chlorella@Fe3O4 surface. When applied for photocatalytic removal of RhB, The CFB biohybrid photocatalyst displayed a 95.6% removal efficiency in 30 min (Xu et al. 2022).
Single-phase magnetite-based nanoparticles are prepared from sole Fe3O4 without the combination of other materials. For example, a study by Kumar et al. (2016) synthesized sphere-shaped Fe3O4 nanoparticles using Andean blackberry leaf extract, where the particles revealed a cubic spinel phase and crystalline structure. When applied for dye-laden wastewater treatment, the nanoparticles displayed a methylene blue photocatalytic degradation rate of 0.0105475 min−1 under sunlight irradiation (Kumar et al. 2016).
Synthesis and characterization techniques of magnetite-based photocatalysts
Synthesis of magnetite-based photocatalysts
Nanomaterial synthesis techniques are selected to yield different shapes and compositions of magnetite-based photocatalysts. Various synthesis methods of photocatalysts and their synthesis procedures are given in Table 2.
The hydrothermal method, sol–gel synthesis technique, and ultrasonication process have been commonly used to prepare magnetite-based photocatalysts.
The hydrothermal synthesis technique involves the use of aqueous media as a reaction system, where wastewater is pressurized and heated to supercritical conditions in a reactor vessel (Bibi et al. 2021). This solution reaction–based approach is properly used to prepare nanomaterials whose precursors are insoluble at room temperature and pressure. It can control the shape, size, particle distribution, and alignment of the synthesized nanomaterials through varying temperatures and pressures.
A two-step hydrothermal method was used to synthesize α-MnO2-Fe3O4 three-dimensional nanoflower-like structure for the treatment of wastewater containing dyes (Rabani et al. 2022). The synthesis process involved the dispersion of Fe3O4 nanoparticles into a solution of potassium permanganate, and the mixture was allowed to stir vigorously. The solution was then transferred to a Teflon-lined stainless-steel autoclave and retained at 150 °C for 3 h and then allowed to cool gradually at room temperature. The nanoflowers were then accumulated as a brown precipitate, which was then washed and dried at 60 °C overnight. When applied for photocatalytic experiments, the nanoflowers displayed high degradation of methylene blue and crystal violet dyes of 94.8% and 93.7%, respectively.
The sol–gel process of nanomaterial preparation involves the use of a solution of the desired nanomaterial precursor called a sol. The sol is then subjected to hydrolysis or condensation, causing an increase in viscosity and converting the solution to the “gel” form (Patil et al. 2021). The gel is further separated into liquid and solid phases by filtration, centrifugation, or sedimentation. Once separated, the solid part is dried to remove the moisture to finally yield the desired nanomaterial (Saeed et al. 2019). The sol–gel method is also simple, economical, and efficient, producing high-quality nanomaterials (Modan and Schiopu 2020). Magnetite–based photocatalysts were successfully synthesized using the sol–gel method and utilized for textile wastewater treatment, depicting dye photocatalytic degradation efficiencies of > 80% in each study (Fauzian et al. 2017; Sadeghi et al. 2021; Wang et al. 2012).
Nanocomposites of Fe3O4/TiO2/Ag were synthesized by suspending TiO2 nanoparticles in a solution of sodium hydroxide followed by stirring at 80 °C (Fauzian et al. 2017). A mixture of Fe3O4 and silver nanoparticles dispersed in a solution of ethanol and distilled water was then added to the suspension and allowed to mix continuously under heating for 2 h. The mixture was then aged to form the gel and then dried at 125 °C for 1 h to obtain the nanocomposite. Applying this nanocomposite for photocatalytic degradation of methylene blue dye gave a high degradation efficiency of 85% within 120 min.
Ultrasonication is also one of the commonly applied nano-photocatalyst synthesis techniques. This method involves the exposure of nanomaterial precursor solutions to ultrasonic irradiation, forming ultrasonic cavitation of the solution (Rane et al. 2018). These developed cavities attempt to continuously absorb and concentrate the diffused sound energy. Sequentially, the cavity bubbles exhibit rapid growth and can no longer absorb the energy efficiently. The liquid will rush into the cavities, leading to cavity implosion. As a result, energy is released to change the solution temperature and pressure, preventing the agglomeration and growth of nanoparticles into larger sizes.
The ultrasonication technique was employed to synthesize a ternary magnetic ZnO/Fe3O4/g-C3N4 composite (Wu et al. 2019). As such, ZnO/Fe3O4 and melamine were ground and dispersed into deionized water, and the formed mixture (with a mass ratio of 1:1) was ultrasonicated for 1 h. The obtained mixture was then dried at 70 °C overnight to remove the solvent and then annealed at 550 °C for 2 h to obtain the nanocomposite. The nanocomposite was applied for treating mono azo dyes-rich wastewater, achieving photocatalytic degradation efficiencies of 97.87%, 83.35%, and 98.05% for methyl orange, orange G, and alizarin yellow R, respectively.
Characterization of magnetite-based photocatalysts
Different types of dyes have been subjected to the photocatalytic degradation process. The behaviour of magnetite-based nanomaterials for photocatalysis could be described using the physical/mechanical, chemical, optical, and electrical parameters. These parameters are determined using the nanomaterial characterization by X-ray diffraction analysis (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and vibrating sample magnetometer (VSM) (Table 3).
The crystalline structure, phase nature, lattice parameters, and crystalline grain size of the fabricated photocatalyst are measured by XRD (Mourdikoudis et al. 2018). Magnetite possesses an inverse spinel cubic structure and conforms to the F3dm JCPDS card (Abdel Maksoud et al. 2020). When a beam of X-rays is directed toward the sample and contacts a crystalline substance/phase, it gets scattered by atoms in the sample (Chhantyal 2022; Sharma et al. 2012). The interference patterns are detected (Chhantyal 2022), and the generated peak characteristics (position and intensity) are compared with the reference patterns available from the International Centre for Diffraction Data (ICDD, previously known as the Joint Committee on Powder Diffraction Standards, JCPDS) database (Mourdikoudis et al. 2018). XRD characterization was performed on the synthesized magnetite-based photocatalysts before and after their application for dyes’ photodegradation (Mazhari et al. 2018; Zhang et al. 2013). In a prepared Fe3O4@SiO2@AgCl/Ag/Ag2S magnetic photocatalyst (Mazhari et al. 2018; Zhang et al. 2013), the diffraction peaks of Fe3O4, AgCl, and Ag2S were assigned by JCPDS, no. 75–0449, JCPDS file: 85–1355, and JCPDS file: 89–3840, respectively. The XRD patterns of Fe3O4@MIL-100(Fe) microspheres complied with the cubic phase Fe3O4 (JCPDS card, file No. 89–4319) and other peaks for the crystalline MIL-100(Fe) (Guidolin et al. 2021).
FTIR provides information about the photocatalyst’s surface molecular composition (Mourdikoudis et al. 2018; Eid 2022). This analysis is carried out by the irradiation of electromagnetic radiation, having wavelengths in the mid-infrared region (4000–400 cm−1), onto the material (Sindhu et al. 2015). The material’s molecules become infrared active once they absorb the radiation, generating an FTIR spectrum (Mourdikoudis et al. 2018). This spectrum displays absorption peaks equivalent to the vibration frequencies of atoms inside the photocatalyst. FTIR has been used as a characterization technique on synthesized magnetite-based nanomaterials (rGO-Fe3O4/TiO2 nanocomposite, TiO2@Fe3O4, ZnO/Fe3O4-SEP, Fe3O4@SiO2/MoO3/PDA-GO composite, Fe3O4/SnO2, Fe3O4@TiO2@PDA/SiW11V-Ag, MOF-1/GO/Fe3O4 nanocomposite, and magnetic BiOBr/Fe3O4/RGO composite), as previously reported (Akkari et al. 2017; Bai et al. 2020; Bibi et al. 2021; Sadeghi et al. 2021; Vasallo-Antonio et al. 2021; Vinosel et al. 2019; Wu et al. 2021; Zheng et al. 2020). For instance, the FTIR spectrum of the synthesized Fe3O4/SiO2/TiO2 composites showed peaks at 800 cm−1 and 1080 cm−1 for Si–O–Si, 940–960 cm−1 for Si–O–Ti, and 500–900 cm−1 for Ti–O–Ti (Wang et al. 2012).
SEM is another technique used to characterize magnetite-based photocatalysts by yielding direct imaging and measurements of the material morphology (Torres-Rivero et al. 2021). SEM also gives information about the material chemical composition (Bastos-Arrieta et al. 2018; Zhou et al. 2006). This technique works by directing a beam of high-energy electrons towards the material to be characterized (Zhou et al. 2006). Once the beam interacts with the material, the material’s electrons are excited and scattered (Zhou et al. 2006). The scattered electrons are received/collected by an Everhart Thornly (ET) detector, which amplifies the electron energies to generate characterization images. The SEM image was used to investigate the microstructure and morphology of a synthesized BiOBr/Fe3O4/RGO composite utilized for rhodamine B dye photodegradation (Zheng et al. 2020). The SEM examination showed that the photocatalytic activity of the composite material was enhanced by the strong connection between the Fe3O4/RGO sheets and BiOBr.
VSM is another characterization method and is used to measure the magnetic moment centres in photocatalysts, following Faraday’s law of induction (Adeyeye and Shimon 2015). The magnetic property is measured by vibrating the material within a constant/uniform magnetic field (often between 50 and 100 Hz) to generate an electric current in suitably positioned sensing coils (Thomson 2014). The sample is connected to a vibrating head module and positioned between two pickup coils (electromagnet poles). The magnetic behaviour of the sample includes saturation magnetization, coercivity, switching fields, and remnant magnetization. The magnetic moment information varies according to the nanoparticles’ morphology, synthetic conditions, shape, and size (Adeyeye and Shimon 2015).
Factors influencing dye removal by magnetite-based photocatalysts
Several operating factors, including substrate pH, dye concentration, and catalyst dosage, influence the efficiency of magnetite-based photocatalysts in degrading textile dyes:
Effect of pH
The solution pH has a great impact on the adsorption properties of the photocatalyst. More positive charges tend to accumulate on the photocatalyst surface when the substrate pH is below the photocatalyst’s pH for the zero-charge point (pHZPC), providing a suitable condition for anionic dye attraction. At pH > pHZPC, the photocatalyst surface gets a net negative charge and attracts the cationic (positively charged) dyes (Alkaim et al. 2014). The pHZPC value can be derived from the pH drift method where the same mass of catalyst solution is suspended in electrolyte solutions of different pH values and a plot of the change in pH (ΔpH) vs. initial pH is generated, revealing the pHZPC as the intersection point. The pHZPC value can also be obtained from the mass titration method, where a concentrated solution of the catalyst material is made by suspending the catalyst in distilled water and the pHZPC is taken as the natural pH of the dispersion; the pHZPC is taken as the intersection point of a graph of inert electrolyte against single titration (Kosmulski 2023). For example, the pHZPC of TiO2 was 6.5, giving the highest photocatalytic efficiency at 7 pH for malachite green and 9 pH for methylene blue (Bibi et al. 2021). As such, the interaction of dyes with the net charge on the photocatalyst surface is also governed by the dye type (either cationic dyes or anionic dyes). Moreover, a highly acidic condition could result in leaching Fe2+ and Fe3+ ions from the magnetite photocatalyst surface into the solution (Abdelhaleem et al. 2019; Guidolin et al. 2021). These leached ions tend to react with a hydrogen peroxide (H2O2) oxidant, following a Fenton-like reaction (Eqs. 9 and 10).
Effect of photocatalysis time
The photocatalytic degradation of dyes in aqueous solution is generally improved by prolonging the reaction time. The photogenerated reactive oxygen species (ROS) and charge carriers get trapped onto the photocatalyst surface and then interact with the dye molecules. The effect of time on dyes’ photodegradation also corresponds to the saturation state of the catalyst surface with dye molecules. Photocatalytic degradation would continue to increase until the catalyst surface becomes saturated, i.e., an approximately horizontal line in a degradation vs. time plot represents a stabilization in the photocatalytic degradation rate.
Effect of catalyst dosage
Figure 3a shows the effect of increasing the photocatalyst dosage on dye removal using data reported in the literature.
The degradation efficiencies are enhanced by increasing the photocatalyst dosage due to the availability of abundant active sites (Abdelhaleem and Chu 2018), increasing the number of free radicals. However, this degradation performance begins to decline after increasing the photocatalyst dosage beyond the optimum value. This pattern was observed in Vasallo-Antonio et al. (2021), where methylene blue degradation of 98.0% was observed at 200 mg/L Fe3O4@SiO2-MoO3-polydopamine-graphene oxide composite photocatalyst under illumination within 60 min. As the catalyst is overdosed, the solution turbidity increases and the light penetration into the solution becomes deficient. This pattern occurs due to reflecting a portion of light from the medium, which reduces the efficiency of hydroxyl radical generation. Increasing the ZnO/biosilica nanocomposite within the 0.1–1.5-g/L range enhanced the decolorization of Acid Red 88 (AR88) from 15.30 to 98.54%, respectively, while this efficiency dropped to 93.63% at a dosage of 2 g/L (Darvishi Cheshmeh Soltani et al. 2015). Increasing the photocatalyst dosage might also be accompanied by particles’ agglomeration, causing loss of the specific surface area and the number of activated sites (Gnanaprakasam et al. 2015).
Effect of initial dye concentration
Figure 3 b reveals a reduction in the photocatalytic degradation efficiencies with increasing the initial dye concentrations (Co), probably due to the inefficient adsorption of O2 and OH− on the photocatalyst. At higher Co, most of the active sites on the photocatalyst become occupied and unable to receive additional dye molecules (Darvishi Cheshmeh Soltani et al. 2015; Salama et al. 2018). Moreover, this condition permits the absorption of a higher percentage of light irradiance on the dye molecules instead of the photocatalyst particles, thereby reducing the catalyst’s potential for ROS generation. In a photodegradation process by composite nanofibers, elevating Co from 10 to 50 ppm reduces the removal efficiencies of different dye compounds from ~ 100 to 70%, respectively (Darvishi Cheshmeh Soltani et al. 2015; Salama et al. 2018). Decreasing the dye degradation efficiency at higher Co was also noticed due to the shortening of the photon path length entering the dye solution (i.e., photons could be blocked before reaching the photocatalyst surface), and the competition between the generated intermediates and dye molecules for the adsorption and photocatalytic sites.
Effect of interfering ions and humic acid
Dye-laden wastewaters usually contain mineral ions, such as Fe2+, Zn2+, Ag+, Na+, Cl−, PO43−, SO42−, BrO3−, CO32−, HCO3−, and S2O82− (Biswas et al. 2022; Rauf and Ashraf 2009), originated from the additives used to improve the textile manufacturing processes. For instance, the CO32− and HCO3− ions are usually added to the dye bath in textile industries for pH adjustment. These ions, based on their concentrations in the medium, attempt to compete with dye molecules for the photocatalytic active sites (Chong et al. 2010). Moreover, some ions, such as Fe2+, CO32−, HCO3−, Cl−, and SO42−, react with OH• radicals (Eqs. 11–13), generating much less reactive ROS (Muruganandham and Swaminathan 2006; Nguyen et al. 2020; Rauf and Ashraf 2009; Rauf et al. 2007). These reactions decrease the HO• radical concentrations, retarding the photocatalysis rate for dye degradation:
The presence of Cl− in the dye solution contributes to hole and hydroxyl radical scavenging effect; i.e., Cl− could react with the photogenerated holes and hydroxyl radicals (Abdelhaleem et al. 2020), where their scavenging effects could be derived in Eqs. 14–17:
The reactions of sulphate ions with the photogenerated holes and •OH radicals in dye-contaminated solution are given by Eqs. 18 and 19 (Abdelhaleem and Chu 2019):
Simultaneously, the ions released in wastewaters may also decline the photocatalytic activity by scavenging electrons, resulting in reduced O2•– radical formation (Rauf et al. 2007).
Persulphate ion (S2O82−) is a strong oxidizing agent that can release sulphate ions in solution, reducing the entire photocatalytic performance (Eq. 20):
The sulphate ions can then react with •OH radicals, as represented in Eq. 19. The sulphate radicals can further react with water molecules to produce more sulphate ions as follows (Eq. 21):
Because SO42− is less reactive than •OH radicals, SO42− accumulation in the medium might occur. This pattern reduces the dye photocatalytic degradation process.
Bromate ion (BrO3−) is an efficient electron scavenger. Therefore, it can react with conduction band electrons e−(CB) in the solution as follows (Eq. 22):
The bromide ions produced in the reaction can interact with •OH radicals (Eq. 23), scavenging them and reducing their concentration in solution (Abdelhaleem et al. 2020; Rauf and Ashraf 2009):
Dye-laden wastewater (with textiles, paints, and varnishes) may also contain humic acids, which can bind to the nanoparticles’ surface (Carlos et al. 2011; Chandran et al. 2014). This binding not only reduces the nanoparticles’ adhesion and agglomeration but also negatively influences the photocatalyst performance. Chandran et al. (2014) showed that humic acid reduced the photocatalytic efficiency of the ZnO nanoparticles (as a photocatalyst), where the rate of MB photodegradation decreased at higher humic acid dosages. A humic acid concentration of 1 mg/L caused blockage to most of the TiO2 active site, reducing the tetracycline photocatalytic removal efficiency (Li and Hu 2016). This insufficient removal pattern could also be assigned to the quenching of ROS (•OH) with humic acid addition.
Degradation and mineralization
The dye photodegradation mechanisms depend mainly on the photocatalyst and ROS types. For instance, the scavenging tests are performed by adding different ROS to the reaction chamber, followed by monitoring the shift in the photocatalytic removal efficiency (Fig. 1).
Benzoquinone (BQ), potassium dichromate (K2Cr2O7), isopropanol (IPA), and ethylenediaminetetraacetate (EDTA-2Na) were employed as the quenchers (trapping agent) of O2•−, e−, •OH, and h+, demonstrating that O2•− was the major ROS for tetracycline degradation by magnetic nanocomposites (Fe3O4/g-C3N4/MoO3) (He et al. 2020). The scavenging test showed a drastic degradation efficiency from 90 to 6% in 120 min after BQ supplementation, whereas adding EDTA-2Na, K2Cr2O7, and IPA resulted in efficiency reductions from 90 to 21%, 39%, and 83%, respectively (Fig. 4).
This test was also performed using sodium oxalate (Na2C2O4), BQ, and IPA to quench h+, O2•−, and •OH, respectively (Xu et al. 2021). In their study, h+ and O2•− were the major reactive species for tetracycline degradation using Fe3O4/BiVO4/CdS heterojunction composite photocatalyst (Xu et al. 2021) (Fig. 4). Based on the heterojunction assumption, the CB position (ECB) of BiVO4 (0.34 eV) was relatively weaker than the O2/O2•− redox potential (− 0.33 eV). Moreover, the VB potential (EVB) of CdS (1.81 eV) was greater than the redox potential of OH−/•OH (+ 1.99 eV) and •OH/H2O (+ 2.38 eV) (Xu et al. 2021). Hence, the Z-scheme path (Fig. 5) could be employed to describe the electron migration from the BiVO4’s CB (0.34 eV) to the CdS’s VB (1.81 eV), followed by recombination with the holes of CdS.
A higher electron–hole separation rate and redox potential support the accumulation of electrons at CdS’s CB, reacting with dissolved O2 to produce O2•−. In parallel, the holes located in the BiVO4’s VB have enough oxidation capacity to (i) react with tetracycline directly and (ii) combine with H2O or OH− to generate •OH.
Mineralization is one of the main objectives of photocatalysis, describing the conversion of organic carbons associated with the dye molecule to CO2 and H2O (Eq. 24):
Mineralization is an essential dye removal pathway because it could mitigate the generation of undesirable toxic intermediate by-products (Zheng et al. 2020). The BiOBr/Fe3O4/RGO photocatalyst showed a strong mineralization performance towards rhodamine B dye removal, where about 91.45% of total organic carbon (TOC) was converted to CO2 and H2O in only 60 min (Zheng et al. 2020). The composite exhibited a promising adsorption–photocatalysis synergistic effect, depicting its applicability for treating industrial effluents without generating secondary pollution.
Real wastewater containing magenta dye was treated by photocatalysis using a TiO2-Fe3O4 composite material, where TOC was monitored before and after degradation (Pucar Milidrag et al. 2022). The decolorization and mineralization efficiencies were about 95% and 85%, respectively, revealing that dye decolorization could not achieve complete oxidation to CO2 and H2O. As such, various intermediates and by-products could be generated in the medium during dye decolorization. Monitoring and identification of such intermediates and by-products are essential not only to identify the removal pathway followed by dye degradation but also to recognize any toxic residuals. Liquid chromatography-tandem mass spectrometry (LC–MS) has been broadly used to exhibit superior and rapid separation efficiency, identifying the composition and structure of these intermediates. Moreover, the degradation pathway during the photocatalytic treatment of wastewater containing dyes could be semi-random, i.e., not following a sole specific route, depending on reaction conditions, and should be studied. Figure 6 shows the degradation mechanisms studied for an azoic dye and a sulphur dye adapted from literature (Touati et al. 2019).
a General degradation by-products of azoic dyes; b degradation mechanism of sulfur black sulfur dye, adapted from Touati et al. (2019)
Management of magnetite-based photocatalysts after textile dye removal
Reusability of magnetite-based photocatalysts
Magnetite-based photocatalysts have displayed several added advantages over other heterogeneous photocatalysts. For example, their magnetic nature allows them to be easily magnetically separated from a solution and reused (Upadhyay et al. 2021) for photocatalytic dye degradation (Table 4). Moreover, magnetic nanomaterials are relatively stable in nature, displaying minimum losses in photocatalytic performance over several cycles, and are able to work within the neutral pH range. For instance, a Fe3O4@SiO2@AgCl/Ag/Ag2S nanocomposite was synthesized and applied for photocatalytic degradation of methyl orange for 10 cycles, after which the degradation efficiency was reduced by only 14% (Mazhari et al. 2018). The textile wastewater treatment stability over 10 successive cycles was validated by revealing minor changes in the chemical composition, surface morphology, crystalline structure, and magnetic performance of the photocatalyst. Also, a ZnO/Fe3O4/g-C3N4 composite photocatalyst was prepared and used to treat wastewater containing methyl orange dye (Wu et al. 2019). After five cycles, the photocatalytic degradation percentage reduced by about 2.57%, and there were insignificant changes in the photocatalyst structure. These findings demonstrate that the number of cycles for photocatalyst reusability could vary according to several factors, such as the solute concentration, cycle period, photocatalyst stability, and material dosage.
Regeneration of magnetite-based photocatalysts
The suitability and reusability of magnetite-based photocatalysts depend on performing a series of pre-treatment steps (Fig. 7) to detach the impurities and excess dye molecules.
The main objective of photocatalyst regeneration is to ensure that any possible blockage of the active sites by the degradation by-products is rectified. A study by Pucar Milidrag et al. (2022) represented the regeneration of a TiO2-Fe3O4 photocatalyst by separation from the reaction medium using an external magnetic field followed by extraction, washing several times with distilled water, and drying in an oven at 105 °C for 2 h. Once dried, the retrieved photocatalyst was then annealed in a muffle furnace at 200 °C for 2 h. Although the regeneration step is essential for removing the impeded impurities, further repeating this procedure might reduce the photocatalytic degradation efficiency due to material scattering and photo-corrosion. A similar study was carried out by Mazhari et al. (2018), regenerating a Fe3O4@SiO2@AgCl/Ag/Ag2S photocatalyst after photodegradation of methyl orange by separation from the dye solution using an external magnetic field. This step was followed by washing with ethanol and deionized water several times and then reusing for photodegradation of a methyl orange-rich solution.
Magnetite-based photocatalysts can also be regenerated by heat treatment. This involves subjecting the used photocatalyst material to high temperatures in order to remove any residual pollutant that may still be present on the catalyst material and causing performance losses (catalyst poisoning). For instance, in a study by (Hossein & Firoozeh 2019), a Fe3O4-PPY-NiO nanocatalyst material was regenerated after each cycle by first magnetically extracting the catalyst from the pollutant solution. This was followed by subjecting the extracted catalyst to heat treatment at 250 °C for 6 h before reapplying it for photocatalytic degradation. After five cycles, he catalyst material displayed a loss in activity of only 20%. Similarly, in a study by Wang et al. (2017), a γ-Fe2O3@SiO2@TiO2 photocatalyst material was subjected to photodegradation of rhodamine B dye for ten consecutive cycles without regeneration. This resulted in a reduction in removal efficiency from nearly 100% in the 1st cycle to about 35% in the tenth cycle. The catalyst material was then regenerated by heat treatment at 200 °C for 30 min. When reapplied for photocatalytic degradation after regeneration, the removal efficiency of the catalyst material was restored to about 100%.
Another catalyst regeneration technique that has been applied to magnetite-based photocatalysts is regeneration by alkaline treatment. In this technique, the catalyst material is washed with a solution of NaOH, dried, and reapplied for photocatalysis. In a study by Idris et al. (2011), the magnetically separable bead catalyst material was regenerated by subjecting the separated catalyst material to washing with a 0.01-M solution of NaOH for 1 h before being reapplied for Cr(VI) removal. The study alluded to the regenerative action of the NaOH to the formation of hydroxides of the pollutant by-products which can then easily detached from the catalyst material, resulting in the unblocking of the catalyst active sites. A γ-Fe2O3/SBA–15/TiO2 photocatalyst material was also regenerated similarly (Yu et al. 2015) after photocatalytic degradation of As(V). The catalyst material was washed in a 0.01-M solution of NaOH and reapplied for photocatalysis, where the catalyst material maintained a degradation efficiency above 90% after five cycles. In this case, the NaOH was able to remove As(V) embedded on the catalyst surface due to the overall negative charge that the catalyst obtains when washed with NaOH. Since arsenic is often present in the form of AsO43-, the negative charge in the catalyst and that on the AsO43- will result in repulsive forces; leading to the desorption of the As from the catalyst surface.
Reuse of treated dye-laden wastewater in the textile industry
Textile manufacturing processes consume large quantities of water, reaching approximately 79 billion cubic meters per annum in 2015 (Pinto et al. 2022). The reuse of treated textile effluents in companies and manufacturing industries for dyeing fabrics is an essential step in maintaining water resource conservation. However, because the effluents of this industry contain dyes, solvents, organic acids, defoamers, reagents/buffers, and washing chemicals, appropriate wastewater treatment methods are required. The quality standards of water used in the textile industry (e.g., for dyeing, cleaning, printing, and finishing) should be better than the water disposal threshold regulations. Wastewater reuse scenarios employed to fulfil the water consumption requirements in the textile finishing industries in Europe were declared (Pinto et al. 2022; Vajnhandl & Valh 2014). Their studies proposed that the integration of multiple treatment technologies (microfiltration and nanofiltration membrane system, coagulation/flocculation/sedimentation, and advanced oxidation processes) was suitable to reduce more than 40% of freshwater consumption by the textile industrial sector. Table S1 lists the applications of treated effluents in the textile industries. Magnetite-based photodegradation processes have displayed sufficient dye and colour removal abilities of up to 100% (Akkari et al. 2017; Sadeghi et al. 2021; Wu et al. 2021) when applied to synthetic and real textile dye-laden waters. Because of their potential to mineralize organic pollutants in waters (Gnanaprakasam et al. 2015; Vinu et al. 2010), magnetite-based photocatalysts could also be used to significantly reduce COD, organic compounds, toxic metals content, and plasticizer content (Khan et al. 2019) of textile wastewaters. The final effluent characteristics are then compared with the allowable ranges before its reuse. Magnetite-based photodegradation is an ideal low-cost, non-toxic, and efficient treatment technique that can be combined with simple treatment processes, such as water softening, pH adjustments, and sedimentation, to treat dye-laden wastewater. The photodegradation effluent can also be reused in various textile processes.
Economic performance of dye removal by magnetite-based photodegradation
The application of magnetite-based photocatalysts for treating dyes-laden wastewater was economically assessed. This step was performed to explore the feasibility of the medium-scale (influent of 10 m3/day) application of the dye-laden wastewater treatment process. The analysis included estimations of capital costs, operating costs, and revenues/earnings.
Capital costs
The initial investments considered in this analysis represented the capital costs of several items. These items shared land footprint, machinery purchases, electrical wiring and piping works, construction expenses, and other amortized costs.
The required volume of the treatment tank (Vc), as calculated by Eq. 25 (Alalm & Nasr 2018), was used to estimate the construction costs:
where Q is the average volume of wastewater treated annually (m3/year), D is the number of working days per year (assuming 300 day/year), tt is the reaction time (e.g., 10 min), and tw is the number of plant operation hours per day (e.g., 720 min/day).
The estimated Vc value was further used to calculate the construction costs (CC) by Eq. 26 (Ansari et al. 2019):
where Cp is the cost price per m3 of a complete photoreactor unit (retrieved from suppliers’ quote).
The capital costs of other items were estimated using CC in the formulae derived from previous studies (Mahamuni & Adewuyi 2010), as given in Table 5. The land purchasing cost was estimated at 1.5% of all other capital investment costs.
Operating costs
The operating costs are used to define the expenses incurred to continuously run the treatment of dyes-laden wastewaters using magnetite-based photocatalysts. These costs are associated with chemicals, electricity, water, repair and maintenance, taxes (statutory obligations), and other periodic expenses. The cost of chemicals was calculated by Eq. 27 (Hamdy et al. 2018):
where Ci is the quantity of chemicals and reagents required per annum (kg/year) and Pi is the cost of the chemical per unit weight ($/kg).
In this calculation, it was assumed that the optimum photocatalyst concentration was 700 mg/L, and it was regenerated and recycled up to 5 times before being carefully discarded and replaced with a fresh catalyst.
The electricity consumption costs were calculated using a consumption rate of 0.16$/kWh (Dadebo et al. 2022). The electricity consumption for treating 1 m3 (electrical energy per order, EEO) of wastewater was computed using the formula given by Eq. 28 (Kumari et al. 2023; Olya et al. 2016). The consumption per m3 and the consumption costs were then used to calculate the annual cost associated with electricity consumption.
where P is the power of all electrical equipment (estimated as 100 W), V is the volume of treated water per day (L), and k is the reaction rate constant (estimated from plot of ln (C/Co) vs. time).
The costs of water utilized to synthesize and regenerate the photocatalyst, prepare an adapted pH medium, and clean the tools and devices are also included in the operating costs. For instance, 300 mL of water, with a water tariff of 0.45$/m3 (Plappally & Lienhard 2012), could be used to prepare 1 g of catalyst. The repair and maintenance costs were estimated at 1.5% of the capital costs (Ansari et al. 2019). The taxes and workers’ remuneration costs were estimated at 1% and 2% of the total capital costs, respectively.
The total operational costs were expressed by electricity consumption (25.64%), chemical utilization (22.43%), water use (4.32%), and others, giving a total running cost of $10.41/m3. A cost analysis (UV light tubes, air sparger, and nano-material preparation) for applying TiO2 in the photocatalytic degradation of Remazol Red dye showed an operating cost of $0.29/L ($290/m3) of treated textile industry effluent (Pipil et al. 2022). In another study (Chawla et al. 2020), the costs of eliminating basic yellow 28 dye using p25-TiO2 and ana-TiO2 were estimated and compared. The operational costs were estimated from the prices of energy and materials, showing Rs. 5090/m3 ($61.09/m3) and Rs. 2900/m3 ($34.80/m3), respectively (Chawla et al. 2020). Another study utilized TiO2 for the photocatalytic removal of pesticides from wastewater (Gar Alalm et al. 2015), revealing an operational COD removal cost of €7.98/m3 ($8.73/m3).
Revenues
The weight of COD removed could be used as an essential criterion to estimate the profits of using the photocatalytic process to reduce environmental pollution. This profit was equivalent to $0.14 for removing a kg COD from industrial effluents, giving 2698.08 $/year. This amount is added to the revenue of the project cash flow, representing economic benefits derived from the initiation of pollution prevention strategies (Ansari et al. 2019). In addition to COD, colour removal was estimated as the removal of nitrogen and phosphorous. Removing 1 kg of each of nitrogen and phosphorous from the textile effluents could add about 8 and 30 $ to the total profits (Ansari et al. 2021). The benefit of onsite reuse of the treated effluent was also added to the project revenue. This value is equivalent to $0.45 per m3 of reused treated water (Dadebo et al. 2022).
Profitability criterion
The economic feasibility of the dye photodegradation process was expressed by the project payback period. The project net profit was estimated from revenue (81,962 $/year) minus operating cost (31,226 $/year), giving 50,736 $/year. The payback time reached about 6.5 year, as calculated from capital cost (330,350 $) ÷ net profit (50,736 $/year). By assessing the cash flow, the revenue gains from pollutants’ removal would recover the prices invested in purchasing the photodegradation project facilities. Moreover, a shorter project payback period (below the project lifetime of 15 year) would be expected due to reusing the exhausted photocatalyst for about five regeneration/reuse cycles.
Achievable sustainable development goals (SDGs) from the photocatalyst technology implementation
The 17 sustainable development goals (SDGs) are a set of goals that include respective targets adopted by the United Nations (UN) member states, achieving world peace and prosperity now and in the future. The goals are integrated to end poverty, improve health and education, reduce inequalities, trigger economic growth, tackle climate change, and preserve oceans and forests. The UN SDGs (Table 6) can be grouped into the social, economic, and environmental pillars of sustainability. This section discusses the SDGs that can be achieved by synthesizing magnetite-based photocatalysis for further applications, including dye-laden wastewater treatment.
Social SDGs
Recent crises, such as the COVID-19 pandemic, rising inflation, and several wars, have elevated the poverty rate and negatively impacted some natural resources such as water. Water is a vital resource required to drive development, and it is crucial for operating various manufacturing industries. For instance, water is utilized at several stages (e.g., bleaching, dyeing, washing, and cooling of equipment) in the textile industry. Magnetite-based photocatalysis could mineralize complex compounds, such as dyes in aqueous solutions, into simpler molecules with less toxicity. This property enables the reclamation of dye-laden wastewater into water of acceptable quality, which can be reused in various human activities, supporting social infrastructure development. Reusing the treated effluents in the industrial sector makes freshwater more available and affordable to society (SDG1: No Poverty). This benefit supports developing countries that do not have access to clean water and sanitation. Recently, several actions have been directed towards resolving food‐web structure and ecosystem function, maintaining SDG2 “Zero Hunger.” For example, reusing the treated effluent in irrigation would boost farming activities and support the agricultural sector.
Moreover, treating the industrial effluents protects the fish species richness from dye toxicity, imposing positive impacts on marine fisheries production and fish farming industries. As such, dye-laden wastewater is toxic to marine life because it slows down photosynthetic processes, reducing the DO levels in the water. This pattern also avoids humans’ exposure to harmful dye chemicals, thereby preventing illness or even death (SDG3: Good health and wellbeing). Scholars and trainees in developing countries can be involved in environmental-related training programs to learn about the applications of wastewater treatment techniques such as magnetite-based photocatalysis (SDG4: Quality Education). Moreover, improved water quality can reduce the risk of waterborne diseases, increasing the number of students (boys and girls) attending classes in the school. The industrial application of magnetite-based photocatalysis for removing dyes can provide job opportunities for women in both low-level and managerial positions (SDG5: Gender Equality). Implementing magnetite-based photocatalysis in low-income nations requires technical assistance and financial aid from more developed countries in accordance with World Trade Organization agreements. This action encourages equality among countries regarding the rights to strengthen and sustain cleaner and greener technologies (SDG10: Reduced inequalities). Transferring the knowledge and skills about advanced wastewater treatment technologies to countries that have complex water security policy challenges is an essential objective for the implication of SDG16 “Peace, justice and strong institutions.” This goal also promotes peaceful relations between the developed and developing nations through improving sustainable planning of the water-food nexus. The formation of international partnerships supports the countries identified with serious water resources shortage and environmental pollution concerns. Moreover, it is essential to maintain strong partnerships between developed and developing nations to alleviate the financial burden related to the initial investment of the magnetite-based nanocomposite manufacturing processes (SDG17: Partnerships for the goals).
Economic SDGs
The manufacturing of magnetite-based photocatalysts and nanocomposites influences several economic-related SDGs. For instance, SDG7, “Affordable and clean energy,” can be endorsed by applying the synthesized nanomaterials in the energy storage and conversion facilities (Muzhanje & Hassan 2023). For instance, phase change materials were enhanced by Al2O3 and CuO nanoparticle supplementation and then studied for their applications towards energy sustainability (harvest-free and/or waste thermal energy). Nanomaterials are also employed in the energy sector organizations by enhancing the solidification, heat transfer, and melting behaviours of the phase change materials (Muzhanje et al. 2023). As such, this approach can address SDG7 by providing efficient, affordable, and sustainable solutions to face the problems associated with conventional heating and cooling systems.
Moreover, the photocatalytic H2 production approach provides a clean and renewable energy source, such as H2 energy application as a future alternative transport fuel. Therefore, photocatalytic nanomaterials can be employed to ensure access to reliable, sustainable, and modern energy, in addition to their existing technologies in wastewater treatment. The applications of photocatalytic technology in environmental safety open more avenues for offering new green jobs (i.e., maintaining SDG8: Decent work and economic growth by employment in environmental-friendly projects). The innovation and industrialization of photocatalysis can open new opportunities for many employees who lost their jobs and companies forced to close down their businesses during the pandemic. Reducing water pollution plays an important role in addressing economic growth and development (SDG9: Industries, innovation and infrastructure). For example, poor water quality reduces labour productivity (health issues), deteriorates the quality and quantity of food production, and negatively impacts the aquaculture and fisheries sectors. Photocatalysis technology can counter the shortfalls associated with conventional methods implementation in wastewater treatment regarding sludge transfer and open dumping in various environmental matrices.
Moreover, wastewater treatment is one of the waste management strategies used to reduce per capita environmental pollution in cities. Addressing these concerns makes cities and human settlements inclusive, safe, resilient, and sustainable (SDG11: Sustainable cities and communities). The strong magnetic property of magnetite-based photocatalysts facilitates their easy separation from the reaction medium, followed by their regeneration and re-application. This property reduces the introduction of new catalysts into the system, promoting sustainable consumption of raw materials. In parallel, this technology lessens the amount of waste generated (i.e., due to material reuse several times), ensuring sustainable consumption and production patterns “SDG12.” Furthermore, the treated effluents could be reused in the textile industry (e.g., cooling of mechanical parts and cleaning and washing of the facilities; see Table S1), reducing the overall water consumption patterns.
Environmental SDGs
Applying magnetite-based photocatalysis for textile dye removal shows great progress in sustaining clean water provision, permitting more people to access clean water and water facilities (SDG6: Clean water and Sanitation). This wastewater treatment approach also attempts to conserve and sustainably protect marine resources and ecology from industrial pollution (SDG14: Life below water) because the dye molecules are absorbed by the fish’s gills and skin, followed by bioaccumulation and biotransformation. Because this process generates fewer quantities of sludge compared with conventional biological systems, it does not cause considerable solid waste pollution (SDG15: Life on Land). As such, the landfilling of textile dyeing sludge poses a threat to the terrestrial environment and ecosystems due to the presence of a wide range of pollutants and hazardous chemicals. The improper consideration of synthetic dyes (e.g., fibres, plastic, polyester, and yarns) increases the possibility of natural habitat degradation and biodiversity loss. Mitigating air pollution by photocatalysis has also been reported, where the CO2 greenhouse gas could be reduced with H2O vapor to generate hydrocarbons and syngas. Converting the greenhouse gas into beneficial sustainable energy fuel (e.g., CH4 and H2) and utilizing the generated fuel to alleviate dependency on fossil fuels are the main routes used to meet the targets of SDG13, “Climate action.”
Barriers facing the application of magnetite-based photocatalysis
Applying magnetite-based photocatalysis to treat dye-laden wastewater has great potential in protecting the environmental dimensions. However, certain challenges should be addressed to fulfil the other pillars of sustainability (economic and social). According to the SDGs concept, these challenges can be grouped as follows (Fig. 8):
Technical barriers
Technical challenges refer to the factors involved in the functionality/complexity, up-scale feasibility, infrastructure, equipment, and other tools used to operate the photocatalysis system. For instance, although magnetite is considered a semiconductor material that possesses good photocatalytic, adsorption, and magnetic properties, its operation suffers from oxidation during generation and cleaning. Combining the magnetite material with other semiconductors or noble metals is one of the technical solutions used to avoid material oxidation, inadequate light utilization, and e−/h+ rapid recombination. However, the semiconductor materials should be rigorously tested and adequately selected because the magnetite phase has the potential to act as a charge carrier recombination centre, reducing the photocatalytic activity of the integrated material. Moreover, the magnetite-based photocatalysis process should be operated under specific pH and time ranges to maintain the best dye degradation schemes within shorter periods, especially for up-scale applications suffering from high fluctuations in wastewater characteristics.
Moreover, employing the same and generalized catalyst to eliminate wide concentrations of different dyes entails technically inaccurate photodegradation performances. For example, a ternary magnetic ZnO/Fe3O4/g-C3N4 composite could degrade about 98% of methyl orange and Alizarin yellow R, but this efficiency was reduced to 83.35% for orange G elimination (Wu et al. 2019). In particular, magnetite-based photocatalysis requires highly skilled and competent personnel for periodic repair, monitoring, and maintenance requirements, ensuring the functionality of all operational parameters at their optimum levels.
Economic barriers
Referring to the cash flow given in Table 5, the operation and maintenance cost for treating 1 m3 of dye-laden wastewater using magnetite-based photocatalysis reached up to $10.41. Although this price is lower than that ($17.745/m3) used to treat dibutyl phthalate-laden wastewater by Fe3O4@PAC as a magnetic nano-composite (Nozari et al. 2022), photocatalysis is still considered an expensive process. As such, magnetite-based photocatalysis is almost 10 times more expensive compared with conventional wastewater treatment techniques, such as anaerobic/aerobic reactors and stabilization ponds ($1.10–$1.46/m3) (Sekandari 2019). Additionally, although solar radiation is abundant in nature and freely available, artificial sources of light (UV radiation) might also be required to possess the photon energy (according to the photocatalyst’s narrow band gap) for efficient dye degradation. Moreover, synthesizing large quantities of magnetite-based photocatalysts for field (up-scale) wastewater treatment applications can be economically infeasible because some of them still need rare or expensive noble metals. The photocatalyst’s manufacturing processes entail complex reactions and require specialized equipment and tools, ultimately raising investment costs (Kuspanov et al. 2023). Despite its recyclability for up to five consecutive runs, this photocatalyst is prone to oxidation and corrosion during handling and transfer. The associated photocatalyst deterioration requires frequent monitoring and maintenance to ensure material effectiveness. Furthermore, some expensive materials such as silica and titanium are used to coat and modify the magnetite-based photocatalysts, increasing their stability for treating industrial effluents under pH-varying conditions.
Environmental barriers
The disposal of exhausted magnetite-based photocatalysts is a major environmental challenge that should be addressed to avoid secondary pollution. As such, this photocatalyst might adsorb some heavy metals and persistent pollutants that could transfer to the environmental domain and then to the human food chain. Thermal treatment (e.g., incineration, pyrolysis, and gasification) is one of the reliable strategies used to manage the utilized photocatalyst. Moreover, the treated effluent should be technically evaluated and characterized for its physicochemical and bacteriological properties because the reuse of treated water should comply with national and international regulations. For instance, the mutagenic and carcinogenic potential of textiles is harmful to human health, and the dye molecules and photocatalyst fine powder could affect plant roots during cultivation with treated textile wastewater, damaging soil quality and plant growth. A study was conducted to understand the exposure effect of magnetite-based nanocomposites on zebrafish embryos and larvae, depicting morphological and physiological alterations at a concentration of 1000 µg/mL (Guillén et al. 2022). However, the genotoxicity of magnetite-based photocatalysts varies depending on the type of material used to prepare the heterogenic composite. Therefore, the risk of toxicity resulting from the accumulation of residual photocatalyst material not separated from the treated effluent should be fully understood before the up-scale application of magnetite-based photocatalysis.
Conclusion and future perspectives
This review succeeded in providing insights into the techno-economic feasibility and sustainability of synthesizing magnetite-based photocatalysts and their applications in textile industry effluent treatment. The dye degradation pathways were explored via electron excitation and transfer, water ionization, oxygen ionosorption, and superoxide protonation. Fe3O4-contained photocatalysts were distinguished by their shape (e.g., spheres, rods, and flowers) and composition (composites, hybrids, or single-phase particles). These types were defined based on the synthesis methods, e.g., hydrothermal, sol–gel, and ultrasonication. The dyes’ removal mechanisms depicted a high correlation with material characterization, regarding the crystalline structure, surface molecular composition, material morphology, and magnetic moment centres. The photocatalytic performance was highly influenced by substrate pH, reaction time, dye concentration, catalyst dosage, and interfering ions and humic acid. The magnetite-based photocatalysts could be regenerated and reused over eight cycles, and the quality of treated effluents could comply with the regulation standards for wastewater reuse in the textile manufacturing industries. For instance, the best magnetite-based photocatalyst was found to be Fe3O4@SiO2@AgCl/Ag/Ag2S nanocomposite as it was recycled up to 10 times under optimal conditions (catalyst dosage = 1200 mg/L, neutral pH, methyl orange concentration = 10 mg/L and time = 60 min).
It should be noted that the composite displayed minimal losses in performance of only 10% ± 4 even after ten consecutive cycles. The study also demonstrated the techno-economic feasibility of scaling up magnetite-based photocatalysis for textile wastewater treatment, giving a payback period shorter than the project’s lifetime. The benefits, as mentioned above, displayed strong interlinkages with the social, economic, and environmental pillars of sustainability, identifying and mitigating the impact of barriers to meet the SDGs strategy.
To increase the feasibility and profitability of the large-scale application of magnetite-based photocatalysis, more research should focus on (i) the possible recycling of the photocatalyst material in the industrial sector for manufacturing magnetic products; (ii) the synthesis of new photocatalysts that could treat dye-rich wastewater under wider pH range, reducing the costs of chemicals required for pH adjustments; (iii) studying the effect of reaction temperature on the performance of magnetite based photocatalysts; (iv) designing cost-effective photoreactors that could utilize natural sunlight for operations, reducing electricity utilization by UV lamps; and (v) getting supports from the international funding agencies to comply with environmental sustainability (e.g., green finance).
Data availability
The data that support the findings of this study are available within the article and/or its supplementary materials.
Code availability
Not applicable.
Abbreviations
- MB :
-
Methylene Blue
- Fe3O4 :
-
Magnetite
- AOP :
-
Advanced oxidation process
- XRD :
-
X-ray diffraction analysis
- FTIR :
-
Fourier Transform infrared spectroscopy
- SEM :
-
Scanning electron microscopy
- VSM :
-
Vibrating sample magnetometer
- TOC :
-
Total organic carbon
- COD :
-
Chemical oxygen demand
- ROS :
-
Reactive oxygen species
- PZC:
-
Point of zero charge
- VB :
-
Valence band
- CB :
-
Conduction band
- rGO:
-
Fe3O4/TiO2 nanocomposite–reduced graphene oxide-based iron oxide modified titania
- 3D α-MnO2-Fe3O4 :
-
Manganese dioxide-incorporated iron oxide three-dimensional nanoflowers
- Fe3O4/TiO2/Ag :
-
Iron oxide and titanium dioxide nanocomposite with additional silver metal
- TiO2@Fe3O4 :
-
Magnetic titanium dioxide nanoparticles
- ZnO/Fe3O4-SEP:
-
Zinc oxide/magnetite-sepiolite nanoplatform
- ZnO/Fe3O4/g-C3N4 :
-
Ternary magnetic zinc oxide/magnetite/graphite-like carbon nitride composite
- g-C3N4/ZnO@Fe3O4 :
-
Graphitic carbon nitride/zinc oxide on magnetite
- Fe3O4@SiO2@TiO2 :
-
Titanium dioxide on silicon dioxide on magnetite
- In2S3/ZnFe2O4 :
-
Indium sulphide/zinc ferrite nanocomposites
- Fe–p-C3N4 :
-
Iron-loaded polymeric carbon nitride
- Fe3O4/SnO2 :
-
Magnetite/tin oxide nanocomposites
- Zn0 . 5Fe3-0.5O4 :
-
Zinc-doped spinel magnetite nanoparticles
- TiO2/Fe3O4 :
-
Titanium dioxide/magnetite nanoparticles
- Fe3O4/BiVO4/CdS:
-
Magnetite/bismuth vanadate/cadmium sulphide heterojunction composite
- Fe3O4/SiO2/TiO2 :
-
Pomegranate-like magnetite/silicon dioxide/titanium dioxide composite microspheres
- Fe3O4/ZnO/Si3N4 :
-
Magnetite/zinc oxide/silicon nitride nanocomposite
- g-C3N4/ZnO@Fe3O4 :
-
Graphitic carbon nitride/zinc oxide on magnetite
- Ch.@Fe3O4@BiOCl microrobots:
-
Ternary biohybrid photocatalytic microrobot based on Chlorella (Ch.) cells
- Fe3O4@TiO2@PDA/SiW11V-Ag:
-
Silver nanoparticles on polydopamine and mono-vanadium substituted silicotungstate co-encapsulated titanium dioxide on magnetite microspheres
- MOF-1/GO/Fe3O4 :
-
Neodymium metal–organic framework/graphene oxide/magnetite nanocomposite
- Magnetic BiOBr/Fe3O4/RGO:
-
Bismuth oxyhalide/magnetite/reduced graphene oxide composite
- Fe3O4@SiO2@AgCl/Ag/Ag2S:
-
Silver chloride/Silver/silver sulphide on silicon dioxide on magnetite core–shell nanocomposite
- Fe3O4@MIL-100(Fe):
-
Metal–organic framework on magnetite core–shell microsphere
- Fe3O4@SiO2/MoO3/PDA-GO:
-
Silicon dioxide on magnetite/molybdenum trioxide/graphene oxide polydopamine matrix
References
Abdel Maksoud, Elgarahy A, Farrell C, Al-Muhtaseb A, Rooney D, Osman A (2020) Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord Chem Rev 1:403. https://doi.org/10.1016/j.ccr.2019.213096
Abdelhaleem A, Chu W (2018) Monuron photodegradation using peroxymonosulfate activated by non-metal-doped TiO2 under visible LED and the modeling via a parallel-serial kinetic approach. J Chem Eng 338:411–421. https://doi.org/10.1016/j.cej.2018.01.036
Abdelhaleem A, Chu W (2019) Insights into peroxymonosulfate activation for carbofuran degradation under visible LED via a double-component photocatalyst of Fe (III) impregnated N-doped TiO2. Chemosphere 12:237. https://doi.org/10.1016/j.chemosphere.2019.124487
Abdelhaleem A, Chu W, Liang X (2019) Diphenamid degradation via sulfite activation under visible LED using Fe (III) impregnated N-doped TiO2 photocatalyst. Appl Catal b: Environmental 244:823–835. https://doi.org/10.1016/j.apcatb.2018.11.085
Abdelhaleem A, Chu W, Farzana S (2020) Diphenamid photodegradation using Fe(III) impregnated N-doped TiO2/sulfite/visible LED process: Influence of wastewater matrix, kinetic modeling, and toxicity evaluation. Chemosphere 256:127094. https://doi.org/10.1016/j.chemosphere.2020.127094
Abdelhaleem A, Abdelhamid H, Ibrahim M, Chu W (2022) Photocatalytic degradation of paracetamol using photo-Fenton-like metal-organic framework-derived CuO@C under visible LED. J Clean Prod 379:134571. https://doi.org/10.1016/j.jclepro.2022.134571
Adeyeye AO, Shimon G (2015) Chapter 1 – Growth and characterization of magnetic thin film and nanostructures. Handb Surf Sci 5:1–41. https://doi.org/10.1016/B978-0-444-62634-9.00001-1
Ahmed S, Haider W (2018) Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: a review. J Nanotechnol 6:29–34. https://doi.org/10.1088/1361-6528/aac6ea
Aich N, Plazas-Tuttle J, Lead J, Saleh N (2014) A critical review of nanohybrids: synthesis, applications, and environmental implications. J Environ Chem 1:11. https://doi.org/10.1071/EN14127
Ajmal A, Majeed I, Malik R, Idriss H, Nadeem M (2014) Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview. RSC Adv 70:37003–37026. https://doi.org/10.1039/C4RA06658H
Akkari M, Aranda P, Mayoral A, García-Hernández M, Amara A, Ruiz-Hitzky E (2017) Sepiolite nanoplatform for the simultaneous assembly of magnetite and zinc oxide nanoparticles as photocatalyst for improving removal of organic pollutants. J Hazard Mater 340:281–290. https://doi.org/10.1016/j.jhazmat.2017.06.067
Alalm M, Nasr M (2018) Artificial intelligence, regression model, and cost estimation for removal of chlorothalonil pesticide by activated carbon prepared from casuarina charcoal. Sustain Environ Res 28:101–110. https://doi.org/10.1016/j.serj.2018.01.003
Alkaim A, Aljeboree A, Alrazaq N, Jaafer S, Hussein F, Lilo A (2014) Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian J Chem 26:8445–8448. https://doi.org/10.14233/ajchem.2014.17908
Ansari F, Ravindran B, Gupta S, Nasr M, Rawat I, Bux F (2019) Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J Environ Manage 240:293–302. https://doi.org/10.1016/j.jenvman.2019.03.123
Ansari F, Nasr M, Rawat I, Bux F (2021) Artificial neural network and techno-economic estimation with algae-based tertiary wastewater treatment. J Water Process Eng 40:101761. https://doi.org/10.1016/j.jwpe.2020.101761
Bai Y, Zhang S, Feng S, Zhu M, Ma S (2020) The first ternary Nd-MOF/GO/Fe3O4 nanocomposite exhibiting an excellent photocatalytic performance for dye degradation. Dalton Trans 49:10745–10754. https://doi.org/10.1039/D0DT01648A
Bastos-Arrieta J, Montes R, Ocaña C, Espinoza M, Muñoz M, Baeza M (2018) In situ characterization of size, spatial distribution, chemical composition, and electroanalytical response of hybrid nanocomposite materials. In: Kumar C (Ed) In-situ characterization techniques for nanomaterials. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-56322-9_8
Behin J, Farhadian N (2016) Response surface methodology and artificial neural network modeling of reactive red 33 decolorization by O3/UV in a bubble column reactor. Adv Environ Technol 2(1):33–44
Benkhaya S, M’rabet S, El Harfi A (2020) Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 6:1. https://doi.org/10.1016/j.heliyon.2020.e03271
Bibi S, Ahmad A, Anjum M, Haleem A, Siddiq M, Shah S, Kahtani A (2021) Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J Environ Chem Eng 9:105580. https://doi.org/10.1016/j.jece.2021.105580
Biswas S, Ghosh S, Maji S, Das S, Roy S, Bhattacharjee R, Mitra P, Malik S, Dey A (2022) Mechanistic aspect of the dye degradation using photocatalysts. In: Dave S, Das J (Eds.) Trends and contemporary technologies for photocatalytic degradation of dyes. Environ Sci Eng pp 247–284, Springer, Cham. Retrieved from https://doi.org/10.1007/978-3-031-08991-6_10
Byrne J, Dunlop P, Hamilton J, Fernández-Ibáñez P, Polo-López I, Sharma P, Vennard A (2015) A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 20:5574–5615. https://doi.org/10.3390/molecules20045574
Carlos L, Cipollone M, Soria D, Moreno M, Ogilby P, García Einschlag F, Mártire D (2011) The effect of humic acid binding to magnetite nanoparticles on the photogeneration of reactive oxygen species. Sep Purif Technol 91:23–29. https://doi.org/10.1016/j.seppur.2011.08.028
Chakraborty JN (2011) Sulphur dyes. In handbook of textile and industrial dyeing: principles, processes and types of dyes. Volume 1 in Woodhead Publishing Series in Textiles. pp 466–485. https://doi.org/10.1533/9780857093974.2.466
Chandran P, Netha S, Khan S (2014) Effect of humic acid on photocatalytic activity of ZnO nanoparticles. J Photochem Photobiol B Biol 138:155–159. https://doi.org/10.1016/j.jphotobiol.2014.05.013
Chawla P, Sharma S, Toor A (2020) Techno-economic evaluation of anatase and p25 TiO2 for treatment basic yellow 28 dye solution through heterogeneous photocatalysis. Environ Dev Sustain 22:231–249. https://doi.org/10.1007/s10668-018-0194-z
Chhantyal P (2022) The use of X-ray diffraction for nanoparticle characterization. AZoOptics, Accessed 27 November 2023, https://www.azooptics.com/Article.aspx?ArticleID=2180
Chong M, Jin B, Chow C, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44:2997–3027. https://doi.org/10.1016/j.watres.2010.02.039
Dadebo D, Nasr M, Fujii M, Ibrahim M (2022) Bio-coagulation using Cicer arietinum combined with pyrolyzed residual sludge-based adsorption for carwash wastewater treatment: a techno-economic and sustainable approach. J Water Process Eng 49:103063. https://doi.org/10.1016/j.jwpe.2022.103063
Darvishi Cheshmeh Soltani R, Khataee AR, Mashayekhi M (2015) Photocatalytic degradation of a textile dye in aqueous phase over ZnO nanoparticles embedded in biosilica nanobiostructure. Desalination Water Treat 57(29):13494–13504. https://doi.org/10.1080/19443994.2015.1058193
dos Santos A, Cervantes F, van Lier J (2007) Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour Technol 98:2369–2385. https://doi.org/10.1016/j.biortech.2006.11.013
Eid MM (2021) Characterization of nanoparticles by FTIR and FTIR-microscopy. In: handbook of consumer nanoproducts. Springer, Singapore. https://doi.org/10.1007/978-981-15-6453-6_89-1
El-Sikaily A, Khaled A, El Nemr A (2012) Textile dyes xenobiotic and their harmful effect. In Non-conventional textile waste water treatment; Nova Science Publishers: New York, NY, USA. pp 31–64
Fauzian M, Taufik A (2017) Photocatalytic performance of Fe O /TiO /Ag nanocomposites for photocatalytic activity under visible light irradiation. AIP Conf Proc 1862, 030031. https://doi.org/10.1063/1.4991135
Gallo-Cordova A, Ovejero J, Pablo-Sainz-Ezquerra A, Cuya J, Jeyadevan B, Veintemillas-Verdaguer S, Tartaj P, Morales M (2022) Unravelling an amine-regulated crystallization crossover to prove single/multicore effects on the biomedical and environmental catalytic activity of magnetic iron oxide colloids. J Colloid Interface Sci 608:1585–1597. https://doi.org/10.1016/j.jcis.2021.10.111
Gar Alalm M, Tawfik A, Ookawara S (2015) Comparison of solar TiO2 photocatalysis and solar photo-Fenton for treatment of pesticides industry wastewater: Operational conditions, kinetics, and costs. J Water Process Eng 8:55–63. https://doi.org/10.1016/j.jwpe.2015.09.007
Gnanaprakasam A, Sivakumar V, Thirumarimurugan M (2015) Influencing parameters in the photocatalytic degradation of organic effluent via nanometal oxide catalyst: a review. Indian J Eng Mater Sci 9:1–16. https://doi.org/10.1155/2015/601827
Guidolin T, Possolli N, Polla M, Wermuth T, Oliveira T, Eller S, Montedo O, Arcaro S, Cechinel M (2021) Photocatalytic pathway on the degradation of methylene blue from aqueous solutions using magnetite nanoparticles. J Clean Prod 318:128556. https://doi.org/10.1016/j.jclepro.2021.128556
Guillén A, Ardila Y, Noguera M, Campaña A, Bejarano M, Akle V, Osma J (2022) Toxicity of modified magnetite-based nanocomposites used for wastewater treatment and evaluated on zebrafish (Danio rerio) model. Nanomaterials 12:3. https://doi.org/10.3390/nano12030489
Hák T, Janoušková S, Moldan B (2016) Sustainable Development Goals: a need for relevant indicators. Ecol Indic 60:565–573. https://doi.org/10.1016/j.ecolind.2015.08.003
Hamdy A, Mostafa M, Nasr M (2018) Zero-valent iron nanoparticles for methylene blue removal from aqueous solutions and textile wastewater treatment, with cost estimation. Water Sci Technol 78:367–378. https://doi.org/10.2166/wst.2018.306
He T, Wu Y, Jiang C, Chen Z, Wang Y, Liu G, Xu Z, Ning G, Chen X, Zhao Y (2020) Novel magnetic Fe3O4/g-C3N4/MoO3 nanocomposites with highly enhanced photocatalytic activities: visible-light-driven degradation of tetracycline from aqueous environment. PLoS ONE 15:1–19. https://doi.org/10.1371/journal.pone.0237389
Hoffmann M, Martin S, Choi W, Bahnemann D (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95(1):69–96. https://doi.org/10.1021/cr00033a004
Hossein F, Firoozeh T (2019) Visible light and UV-assisted photodegradation of phenylephrine by bulk NiO and NiO immobilized on magnetic polypyrrole support. Int J Pharm Sci Dev Res 5(1):06–024
Hussain S, Khan N, Gul S, Khan S, Khan H (2020) Contamination of water resources by food dyes and its removal technologies. IntechOpen. https://doi.org/10.5772/intechopen.90331
Idris A, Hassan N, Rashid R, Ngomsik AF (2011) Kinetic and regeneration studies of photocatalytic magnetic separable beads for chromium (VI) reduction under sunlight. J Hazard Mater 186(1):629–635
Karim M, Aziz K, Omer K, Salih Y, Mustafa F, Rahman K, Mohammad Y (2022) Degradation of aqueous organic dye pollutants by heterogeneous photo-assisted Fenton-like process using natural mineral activator: parameter optimization and degradation kinetics. IOP Conf Ser Earth Environ Sci 958:1. https://doi.org/10.1088/1755-1315/958/1/012011
Khan S, Sayed M, Sohail M, Shah LA, Raja MA (2019) Advanced oxidation and reduction processes. Advances in water purification techniques. Elsevier pp 135-164. https://doi.org/10.1016/B978-0-12-814790-0.00006-5
Kiron M (2013). Properties, classification and application of basic dyes. Textile Learner. https://textilelearner.net/basic-dyes-properties-classification/. Accessed 26 November 2023
Kiron M (2021a) Disperse dyes: properties, classification, dyeing & printing method. Textile Learner. https://textilelearner.net/disperse-dyes-dyeing-and-printing-method/. Accessed 26 November 2023
Kiron M (2021b) Reactive dyes: classification, dyeing mechanism, application & stripping. Textile Learner. https://textilelearner.net/reactive-dyes-classification-dyeing-mechanism/. Accessed 26 November 2023
Kosmulski M (2023) The pH dependent surface charging and points of zero charge. X Update Adv Colloid Interface SCI 319 Article 102973. https://doi.org/10.1016/j.cis.2023.102973
Kumar B, Smita K, Cumbal L, Debut A, Galeas S, Guerrero V (2016) Phytosynthesis and photocatalytic activity of magnetite (Fe3O4) nanoparticles using the Andean blackberry leaf. Mater Chem Phys 179:310–315. https://doi.org/10.1016/j.matchemphys.2016.05.045
Kumar A, Dixit U, Singh K et al (2021) Structure and properties of dyes and pigments. Dyes and pigments - novel applications and waste treatment. IntechOpen. Available at: http://doi.org/10.5772/intechopen.97104
Kumari H, Sonia S, Ranga R, Chahal S, Devi S, Sharma S, Kumar S, Kumar P, Kumar S, Kumar A, Parmar R (2023) A review on photocatalysis used for wastewater treatment: dye degradation. Wat Air and Soil Poll 234:6. https://doi.org/10.1007/s11270-023-06359-9
Kuspanov Z, Bakbolat B, Baimenov A, Issadykov A, Yeleuov M, Daulbayev C (2023) Photocatalysts for a sustainable future: innovations in large-scale environmental and energy applications. Sci Total Environ 885:8. https://doi.org/10.1016/j.scitotenv.2023.163914
Lellis B, Fávaro-Polonio C, Pamphile J, Polonio J (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov 3:275–290. https://doi.org/10.1016/j.biori.2019.09.001
Li S, Hu J (2016) Photolytic and photocatalytic degradation of tetracycline: effect of humic acid on degradation kinetics and mechanisms. J Hazard Mater 318:134–144. https://doi.org/10.1016/j.jhazmat.2016.05.100
Lian S, Kang Z, Wang E, Jiang M, Hu C, Xu L (2003) Convenient synthesis of single crystalline magnetic Fe3O4 nanorods. Solid State Commun 127:605–608. https://doi.org/10.1016/S0038-1098(03)00580-5
Mahamuni N, Adewuyi Y (2010) Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: a review with emphasis on cost estimation. Ultrason Sonochem 17:990–1003. https://doi.org/10.1016/j.ultsonch.2009.09.005
Manohar A, Chintagumpala K, Kim K (2021) Magnetic hyperthermia and photocatalytic degradation of rhodamine B dye using Zn-doped spinel Fe3O4 nanoparticles. J Mater Sci Mater Electron 32:8778–8787. https://doi.org/10.1007/s10854-021-05549-7
Mazhari MP, Hamadanian M (2018) Preparation and characterization of Fe3O4@SiO2@TiO2 and Ag/Fe3O4@SiO2@TiO2 nanocomposites for water treatment: process optimization by response surface methodology. J Electron Mater 47:7484–7496. https://doi.org/10.1007/s11664-018-6690-y
Mazhari MP, Hamadanian M, Mehipour M, Jabbari V (2018) Central composite design (CCD) optimized synthesis of Fe3O4@SiO2@AgCl/Ag/Ag2S as a novel magnetic nano-photocatalyst for catalytic degradation of organic pollutants. J Environ Chem Eng 6:7284–7293. https://doi.org/10.1016/j.jece.2018.11.024
Mercyrani B, Hernandez-Maya R, Solís-López M, Th-Th C, Velumani S (2018) Photocatalytic degradation of Orange G using TiO2/Fe3O4 nanocomposites. J Mater Sci Mater Electron 29:15436–15444. https://doi.org/10.1007/s10854-018-9069-1
Pucar Milidrag G, Nikić J, Gvoić V, Kulić Mandić A, Agbaba J, Bečelić-Tomin M, Kerkez D (2022) Photocatalytic degradation of magenta effluent using magnetite doped TiO in solar parabolic trough concentrator. Catalysts 12(9):986. https://doi.org/10.3390/catal12090986
Mishra P, Patnaik S, Parida K (2019) An overview of recent progress on noble metal modified magnetic Fe3O4 for photocatalytic pollutant degradation and H2 evolution. Catal Sci Technol 9:916–941. https://doi.org/10.1039/C8CY02462F
Modan E, Schiopu A (2020) Advantages and disadvantages of chemical methods in the elaboration of nanomaterials. J Metall Mater Sci 43(6):53–60. https://doi.org/10.35219/mms.2020.1.08
Moradi Z, Jahromi S, Ghaedi M (2021) Design of active photocatalysts and visible light photocatalysis. Interf Sci Technol 32:557–623. https://doi.org/10.1016/B978-0-12-818806-4.00012-7
Mourdikoudis S, Pallares R, Thanh N (2018) Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 10:12871–12934. https://doi.org/10.1039/C8NR02278J
Muruganandham M, Swaminathan M (2006) Photocatalytic decolourisation and degradation of Reactive Orange 4 by TiO2-UV process. Dyes Pigm 68:133–142. https://doi.org/10.1016/j.dyepig.2005.01.004
Muzhanje AT, Hassan H (2023) Microcapsule geometry and nanomaterial enhancement of PCMs (sp07&sp11) for free heating applications. Case Stud Therm Eng 49:103327. https://doi.org/10.1016/j.csite.2023.103327
Muzhanje AT, Ookawara S, Hassan H, Hassan MA (2023) The heat transfer with nanomaterial enhanced phase change materials in different container shapes. J Energy Syst 7:173–186. https://doi.org/10.30521/jes.1160434
Nguyen T, Nam S, Kim J, Oh J (2020) Photocatalytic degradation of dissolved organic matter under ZnO-catalyzed artificial sunlight irradiation system. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-69115-7
Nozari M, Malakootian M, Fard N, Mahmoudi-Moghaddam H (2022) Synthesis of Fe3O4@PAC as a magnetic nano-composite for adsorption of dibutyl phthalate from the aqueous medium: Modeling, analysis and optimization using the response surface methodology. Surf Interfaces 31:101981. https://doi.org/10.1016/j.surfin.2022.101981
Ogugbue C, Sawidis T (2011) Bioremediation and detoxification of synthetic wastewater containing triarylmethane dyes by Aeromonas hydrophila isolated from industrial effluent. Biotechnol Res Int 7:1–11. https://doi.org/10.4061/2011/967925
Olya M, Akhi Y, Marandi R (2016) Improvement of efficiency and electrical energy consumption of AB74 degradation process using a novel cylindrical batch photochemical reactor. Orient J Chem 32:1295–1303. https://doi.org/10.13005/ojc/320303
Osman A, Elgarahy A, Mehta N, Al-Muhtaseb A, Al-Fatesh A, Rooney D (2022) Facile synthesis and life cycle assessment of highly active magnetic sorbent composite derived from mixed plastic and biomass waste for water remediation. ACS Sustain Chem Eng 10:12433–12447. https://doi.org/10.1021/acssuschemeng.2c04095
Osman A, Elgarahy A, Eltaweil A, Abd El-Monaem E, El-Aqapa H, Park Y, Hwang Y, Ayatl A, Farghall M, Ihara I, Al-Muhtaseb A, Rooney D, Yap PS, Sillanpää M (2023) Biofuel production, hydrogen production and water remediation by photocatalysis, biocatalysis and electrocatalysis. Environ Chem Lett 21:315–1379. https://doi.org/10.1007/s10311-023-01581-7
Pandit K, Jeffrey C, Keogh J, Tiwari MS, Artioli N, Manyar HG (2023) Techno-Economic Assessment and Sensitivity Analysis of Glycerol Valorization to Biofuel Additives via Esterification. Ind Eng Chem Res 62:9201–9210
Patil N, Bhaskar R, Vyavhare V, Dhadge R, Khaire V, Patil Y (2021) Overview on methods of synthesis of nanoparticles. Int J Curr Pharm Res 13:11–16. https://doi.org/10.1016/j.jddst.2019.101174
Pinto C, Fernandes A, Lopes A, Nunes M, Baía A, Ciríaco L, Pacheco M (2022) Reuse of textile dyeing wastewater treated by electrooxidation. Water (Switzerland) 14:7. https://doi.org/10.3390/w14071084
Pipil H, Yadav S, Chawla H, Taneja S, Verma M, Singla N, Haritash A (2022) Comparison of TiO2 catalysis and Fenton’s treatment for rapid degradation of Remazol Red Dye in textile industry effluent. Rend Fis Acc Lincei 33:105–114. https://doi.org/10.1007/s12210-021-01040-x
Plappally AK, Lienhard JH (2012) Costs for water supply, treatment, end-use and reclamation. Desalination and Water Treatment. Desalin Water Treat 51:1–33. https://doi.org/10.1080/19443994.2012.708996
Rabani I, Bathula C, Zafar R, Tahi M, Park YJ, Kim HS, Naushad M, Seo YS (2022) Visible light-driven photocatalytic rapid degradation of organic contaminants engaging manganese dioxide-incorporated iron oxide three dimensional nanoflowers. Colloid Interface Sci 608:2347–2357. https://doi.org/10.1016/j.jcis.2021.10.149
Rajamohan S, Kumaravel V, Muthuramalingam R, Ayyadurai S, Abdel-Wahab A, Sub Kwak B, Kang M, Sreekantan S (2017) Fe3O4-Ag2WO4: Facile synthesis, characterization and visible light assisted photocatalytic activity. New J Chem 41:11722–11730. https://doi.org/10.1039/C7NJ03004E
Rane A, Kanny K, Abitha V, Thomas S, Thomas S (2018) Chapter 5 - methods for synthesis of nanoparticles and fabrication of nanocomposites. S. Mohan Bhagyaraj, O.S. Oluwafemi, N. Kalarikkal, S. Thomas (Eds.), Synthesis of inorganic nanomaterials, Woodhead Publishing, pp 121–139. https://doi.org/10.1016/B978-0-08-101975-7.00005-1
Rauf M, Ashraf S (2009) Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. J Chem Eng 151:10–18. https://doi.org/10.1016/j.cej.2009.02.026
Rauf M, Bukallah S, Hamadi A, Sulaiman A, Hammadi F (2007) The effect of operational parameters on the photoinduced decoloration of dyes using a hybrid catalyst V2O5/TiO2. J Chem Eng 129:167–172. https://doi.org/10.1016/j.cej.2006.10.031
Ren G, Han H, Wang Y, Liu S, Zhao J, Meng X, Li Z (2021) Recent advances of photocatalytic application in water treatment: a review. J Nanomater 11:7. https://doi.org/10.3390/nano11071804
Sadeghi M, Heydari M, Javanbakht V (2021) Photocatalytic and photo-fenton processes by magnetic nanophotocatalysts for efficient dye removal. J Mater Sci Mater Electron 32:5065–5081. https://doi.org/10.1007/s10854-021-05241-w
Saeed T, Naeem A, Mahmood T, Huma Khan N (2020) Preparation of nano-particles and their applications in adsorption. IntechOpen. https://doi.org/10.5772/intechopen.89534
Said B, Souad MR, Ahmed E (2020) A review on classifications, recent synthesis and applications of textile dyes. Inorg Chem Commun 3:107891. https://doi.org/10.1016/j.inoche.2020.107891
Salama A, Mohamed A, Aboamera N, Osman T, Khattab A (2018) Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Appl Nanosci 8:155–161. https://doi.org/10.1007/s13204-018-0660-9
Sekandari A (2019) Cost comparison analysis of wastewater treatment plants. Int J Eng Sci 6:2349–784X. http://www.ijste.org/articles/IJSTEV6I1021.pdf
Sharma G, Kumar A, Sharma S, Naushad M, Dhiman P, Vo DV, Stadler F (2020) Fe3O4/ZnO/Si3N4 nanocomposite based photocatalyst for the degradation of dyes from aqueous solution. Mater Lett 278:128359. https://doi.org/10.1016/j.matlet.2020.128359
Sharma R, Bisen D, Shukla U, Sharma B (2012) X-ray diffraction: a powerful method of characterizing nanomaterials. Recent Res Sci Technol 4:77–79. https://updatepublishing.com/journal/index.php/rrst/article/view/933
Sindhu R, Bino, P, Pandey A (2015) Chapter 17 - Microbial poly-3-hydroxybutyrate and related copolymers. A. Pandey, R. Höfer, M. Taherzadeh, K.M. Nampoothiri, C. Larroche (Eds.), Industrial biorefineries & white miotechnology, Elsevier, Amsterdam, pp 575-605. https://doi.org/10.1016/B978-0-444-63453-5.00019-7
Tawfik A, Alalm MG, Awad HM, Islam M, Qyyum MA, Al-Muhtaseb AH, Osman A, Lee M (2022) Solar photo-oxidation of recalcitrant industrial wastewater: a review. Environ Chem Lett 20:1839–1862. https://doi.org/10.1007/s10311-022-01390-4
Thomson T (2014) 10 - Magnetic properties of metallic thin films. K. Barmak, K. Coffey (Eds.), Metallic films for electronic, optical and magnetic applications, Woodhead Publishing, pp 454–546. https://doi.org/10.1533/9780857096296.2.454
Torres-Rivero K. Bastos-Arrieta J, Fiol N, Florido A (2021) Chapter Ten - Metal and metal oxide nanoparticles: an integrated perspective of the green synthesis methods by natural products and waste valorization: applications and challenges. In: Verma S, Das A (Eds.) Biosynthesized Nanomaterials, 94:433–469.
Touati A, Jlaiel L, Najjar W, Sayadi S (2019) Photocatalytic degradation of sulfur black dye over Ce-TiO2 under UV irradiation: removal efficiency and identification of degraded species. Euro-Mediterr J Environ Integr 4:1. https://doi.org/10.1007/s41207-018-0086-5
Upadhyay P, Moholkar VS, Chakma S (2021) Chapter 9 - Magnetic nanomaterials-based photocatalyst for wastewater treatment. In: Bhanvase B, Sonawane S, Pawade V, Pandit A (Eds) Handbook of Nanomaterials for Wastewater Treatment: Fundamentals and Scale Up Issues, pp 241–27. https://doi.org/10.1016/B978-0-12-821496-1.00008-8
Vajnhandl S, Valh J (2014) The status of water reuse in European textile sector. J Environ Manage 141:29–35. https://doi.org/10.1016/j.jenvman.2014.03.014
Vasallo-Antonio R, Peña-Bahamonde J, Susman M, Ballesteros F, Rodrigues D (2021) Design and performance of Fe3O4@SiO2/MoO3/polydopamine-graphene oxide composites for visible light photocatalysis. Emergent Mater 4:1425–1439. https://doi.org/10.1007/s42247-020-00142-w
Vinosel V, Anand S, Janifer M, Pauline S, Dhanavel S, Praveena P, Stephen A (2019) Enhanced photocatalytic activity of Fe3O4/SnO2 magnetic nanocomposite for the degradation of organic dye. J Mater Sci Mater Electron 30:9663–9677. https://doi.org/10.1007/s10854-019-01300-5
Vinu R, Akki S, Madras G (2010) Investigation of dye functional group on the photocatalytic degradation of dyes by nano-TiO2. J Hazard Mater 176:765–773. https://doi.org/10.1016/j.jhazmat.2009.11.101
Vu V, Bartling S, Peppel T, Lund H, Kreyenschulte C, Rabeah J, Moustakas NG, Sukrus AE, Ta HG, Steinfeldt N (2020) Enhanced photocatalytic performance of polymeric carbon nitride through combination of iron loading and hydrogen peroxide treatment. Colloids Surf a: Physicochem Eng 589:124383. https://doi.org/10.1016/j.colsurfa.2019.124383
Wang F et al (2017) Corn-like, recoverable γ-Fe2O3@SiO2@TiO2 photocatalyst induced by magnetic dipole interactions. Sci Rep 12(7):1
Wang R, Wang X, Xi X, Hu R, Jiang G (2012) Preparation and photocatalytic activity of magnetic FeO /SiO /TiO composites. Adv Mater Sci Eng. Article ID 409379. https://doi.org/10.1155/2012/409379
Weng B, Qi MY, Han C, Tang ZR, Xu YJ (2019) Photocorrosion inhibition of semiconductor-based photocatalysts: basic principle, current development, and future perspective. ACS Catal 9:4642–4687. https://doi.org/10.1021/acscatal.9b00313
Wu Z, Chen X, Liu X, Yang X, Yang Y (2019) A ternary magnetic recyclable ZnO/Fe3O4/g-C3N4 composite photocatalyst for efficient photodegradation of Monoazo dye. Nanoscale Res Lett 14:147. https://doi.org/10.1186/s11671-019-2974-2
Wu P, Xue Q, Liu J, Wang T, Feng C, Liu B, Hu H, Xue G (2021) In situ depositing Ag NPs on PDA/SiW11V Co-encapsulated Fe3O4@TiO2 magnetic microspheres as highly efficient and durable visible-light-driven photocatalysts. Chem Cat Chem 13:388–396. https://doi.org/10.1002/cctc.202001539
Xu G, Du M, Li T, Guan Y, Guo C (2021) Facile synthesis of magnetically retrievable Fe3O4/BiVO4/CdS heterojunction composite for enhanced photocatalytic degradation of tetracycline under visible light. Sep Purif Technol 275:119157. https://doi.org/10.1016/j.seppur.2021.119157
Xu L, Gong D, Celi N, Xu J, Zhang D, Cai J (2022) Biohybrid magnetic microrobots for enhanced photocatalytic RhB degradation and E. coli inactivation under visible light irradiation. Appl Surf Sci 579:152165. https://doi.org/10.1016/j.apsusc.2021.152165
Yang S, Sun Q, Shen Y, Hong Y, Tu X, Chen Y, Zheng H (2020) Design, synthesis and application of new iron-based cockscomb-like photocatalyst for high effectively degrading water contaminant under sunlight. Appl Surf Sci 525:146559. https://doi.org/10.1016/j.apsusc.2020.146559
Yu L, Yang X, Wang D (2015) TiO2 incorporated in magnetic mesoporous SBA-15 by a facile inner-pore hydrolysis process toward enhanced adsorption-photocatalysis performances for As(III). J Colloid Interface Sci 6(448):525–532
Zhang CF, Qiu LG, Ke F, Zhu YJ, Yuan YP, Xu GS, Jiang X (2013) A novel magnetic recyclable photocatalyst based on a core–shell metal–organic framework Fe3O4@MIL-100(Fe) for the decolorization of methylene blue dye. J Mater Chem A 1:14329–14334. https://doi.org/10.1039/C3TA13030D
Zhao W, Huang Y, Su C, Gao Y, Tian W, Yang X (2020) Fabrication of magnetic and recyclable In2S3/ZnFe2O4 nanocomposites for visible light photocatalytic activity enhancement. Mater Res Express 7:15080. https://doi.org/10.1088/2053-1591/ab6aca
Zheng M, Ma X, Hu J, Zhang X, Li D, Duan W (2020) Novel recyclable BiOBr/Fe3O4/RGO composites with remarkable visible-light photocatalytic activity. RSC Adv 10:19961–19973. https://doi.org/10.1039/D0RA01668C
Zhou W, Apkarian R, Wang ZL, Joy D (2006) Fundamentals of scanning electron microscopy (SEM). In: Zhou W, Wang ZL (eds) scanning microscopy for nanotechnology. Springer, New York, NY. https://doi.org/10.1007/978-0-387-39620-0_1
Zhu M, Diao G (2011) Synthesis of porous Fe3O4 nanospheres and its application for the catalytic degradation of xylenol orange. J Phys Chem C 115:18923–18934. https://doi.org/10.1021/jp200418j
Acknowledgements
The authors are very grateful to the JICA-Japan International Cooperation Agency for providing all facilities and equipment to accomplish this study. The first author is very grateful to TICAD7 African Scholarship.
Funding
The first author received financial support from the TICAD7 and Egypt-Japan University of Science and Technology (E-JUST) in the form of an MSc scholarship. The third author received support from The Bryden Centre project (Project ID VA5048). The Bryden Centre project is supported by the European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB).
Author information
Authors and Affiliations
Contributions
KN: methodology, formal analysis, writing — original draft; AA: supervision, conceptualization, writing — review and editing; AO: conceptualization, visualization, writing — review and editing; LP: writing — review and editing; MN: supervision, conceptualization, visualization, writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Disclaimer
The views and opinions expressed in this review do not necessarily reflect those of the European Commission or the Special EU Programs Body (SEUPB).
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ngulube, K., Abdelhaleem, A., Osman, A.I. et al. Advancing sustainable water treatment strategies: harnessing magnetite-based photocatalysts and techno-economic analysis for enhanced wastewater management in the context of SDGs. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-32680-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-32680-9