Abstract
Although Mn(III) complexes with organic ligands have been previously identified, the information about their stability and reactivity is scarce. In the present study, we analyzed the formation and stability of three different complexes: Mn(III)-citrate, Mn(III)-tartrate, and Mn(III)-humic acid (HA), as well as their reactivity toward an element of high environmental concern, lead (Pb).
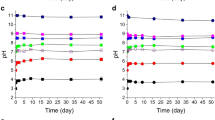
Our results indicate that the stability of studied complexes is highly dependent on pH. The Mn(III) complexes with citrate and tartrate degrade below pH 8, due to the electron transfer reaction between Mn(III) and the ligand, while the Mn(III)-HA complex’s degradation is slower and less sensitive to pH. At pH 4, less than 40% of the initial Mn(III)-HA was found to be stable.
The reactivity of the complexes was different depending on the ligand and its concentration. The Mn(III)-citrate and Mn(III)-tartrate complexes effectively reduced PbO2 and releases aqueous Pb2+, although significant differences were found with increasing ligand concentration. There was no evidence of the reduction of PbO2 by Mn(III) when it forms a complex with HA. This is likely due to the large size of HA moieties that prevent the Mn(III) component of the complex from getting close enough to the PbO2 surface to initiate electron transfer and lead to the reduction of Pb(IV) by HA itself.
Graphical Abstract






Similar content being viewed by others
Data availability
All the data and software used in this manuscript comply with field standards. All the datasets can be made available upon request.
References
Adeleke R, Nwangburuka C, Oboirien B (2017) Origins, roles and fate of organic acids in soils: a review. S Afr J Bot 108:393–406
Bernaldo BG, Bertsch F, Nilo GP, Suvannang N, Hayr De R (2019) Standard operating procedure for soil organic carbon Walkley-Black method titration and colorimetric method. United Nations Food and Agriculture Organization (GLOSOLAN-SOP-02)
Bode AAC, Granneman SJC, Feiters MC, Verwer P, Jiang S, Meijer JAM, van Enckevort WJP, Vlieg E (2016) Structure and activity of the anticaking agent iron(iii) meso-tartrate. Dalton Trans 45:6650–6659
de Melo BAG, Motta FL, Santana MHA (2016) Humic acids: structural properties and multiple functionalities for novel technological developments. Mater Sci Eng, C 62:967–974
Dryer DJ, Korshin GV (2007) Investigation of the reduction of lead dioxide by natural organic matter. Environ Sci Technol 41:5510–5514
Gates-Rector S, Blanton T (2019) The Powder Diffraction File: a quality materials characterization database. Powder Diffr 34:352–360
Gautier-Luneau I, Merle C, Phanon D, Lebrun C, Biaso F, Serratrice G, Pierre J-L (2005) New trends in the chemistry of Iron(III) citrate complexes: correlations between X-ray structures and solution species probed by electrospray mass spectrometry and kinetics of iron uptake from citrate by iron chelators. Chem - Eur J 11:2207–2219
Haynes W (2014) CRC handbook of chemistry and physics. CRC Press, Boca Raton, p 95
Jones MR, Luther GW, Mucci A, Tebo BM (2019) Concentrations of reactive Mn(III)-L and MnO2 in estuarine and marine waters determined using spectrophotometry and the leuco base, leucoberbelin blue. Talanta 200:91–99
Klewicki JK, Morgan JJ (1998) Kinetic behavior of Mn(III) complexes of pyrophosphate, EDTA, and citrate. Environ Sci Technol 32:2916–2922
Kostka JE, Luther GW, Nealson KH (1995) Chemical and biological reduction of Mn (III)-pyrophosphate complexes: potential importance of dissolved Mn(III) as an environmental oxidant. Geochim Cosmochim Acta 59:885–894
Li Q, Xie L, Jiang Y, Fortner JD, Yu K, Liao P, Liu C (2019) Formation and stability of NOM-Mn(III) colloids in aquatic environments. Water Res 149:190–201
Lin Y-P, Washburn MP, Valentine RL (2008) Reduction of lead oxide (PbO2) by iodide and formation of iodoform in the PbO2/I−/NOM system. Environ Sci Technol 42:2919–2924
Lipps WC, Braun-Howland EB, Baxter TE (eds) (2023) Standard methods for the examination of water and wastewater, 24th edn. APHA Press, Washington DC
Namgung S, Guo B, Sasaki K, Lee SS, Lee G (2020) Macroscopic and microscopic behaviors of Mn(II) (ad)sorption to goethite with the effects of dissolved carbonates under anoxic conditions. Geochim Cosmochim Acta 277:300–319
Noel JD, Wang Y, Giammar DE (2014) Effect of water chemistry on the dissolution rate of the lead corrosion product hydrocerussite. Water Res 54:237–246
Qian A, Zhang W, Shi C, Pan C, Giammar DE, Yuan S, Zhang H, Wang Z (2019) Geochemical stability of dissolved Mn(III) in the presence of pyrophosphate as a model ligand: complexation and disproportionation. Environ Sci Technol 53:5768–5777
Saito T, Koopal LK, Van Riemsdijk WH, Nagasaki S, Tanaka S (2004) Adsorption of humic acid on goethite: isotherms, charge adjustments, and potential profiles. Langmuir 20:689–700
Shi Z, Stone AT (2009) PbO2(s, Plattnerite) Reductive dissolution by natural organic matter: reductant and inhibitory subfractions. Environ Sci Technol 43:3604–3611
Szlamkowicz I, Stanberry J, Lugo K, Murphy Z, Ruiz Garcia M, Hunley L, Qafoku NP, Anagnostopoulos V (2023) Role of manganese oxides in controlling subsurface metals and radionuclides mobility: a review. ACS Earth and Space Chemistry 7:1–10
Topolski A (2011) Insight into the degradation of a manganese(III)-citrate complex in aqueous solutions. Chem Pap 65:389–392
Wang X, Wang Q, Yang P, Wang X, Zhang L, Feng X, Zhu M, Wang Z (2020) Oxidation of Mn(III) Species by Pb(IV) oxide as a surrogate oxidant in aquatic systems. Environ Sci Technol 54:14124–14133
Wang Y, Xie Y, Li W, Wang Z, Giammar DE (2010) Formation of lead(IV) oxides from lead(II) compounds. Environ Sci Technol 44:8950–8956
Wang Z, Xiong W, Tebo BM, Giammar DE (2014) Oxidative UO2 dissolution induced by soluble Mn(III). Environ Sci Technol 48:289–298
Webb SM, Dick GJ, Bargar JR, Tebo BM (2005) Evidence for the presence of Mn(III) intermediates in the. Proc Natl Acad Sci 102:5558–5563
Acknowledgements
The authors are thankful to the Materials Characterization Facility (AMPAC) at University of Central Florida for the XRD analysis. The authors are grateful to Ilana Szlamkowicz and Zachary Murphy for corrections of the manuscript and to Dr. Jonathan Caranto for his assistance with EPR measurements.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by Mismel Ruiz Garcia, Mark Richards, and Giovanna Ballerini Ribeiro Gomes. The first draft of the manuscript was written by Mismel Ruiz Garcia, and all the authors commented on the previous versions of the manuscript. Conceptualization was performed by Vasileios Anagnostopoulos and Mismel Ruiz Garcia.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruiz-Garcia, M., Richards, M., Ballerini Ribeiro Gomes, G. et al. PbO2 reductive dissolution by dissolved Mn(III) in the presence of low molecular weight organic acids and humic acid. Environ Sci Pollut Res 31, 18540–18548 (2024). https://doi.org/10.1007/s11356-024-32319-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32319-9