Abstract
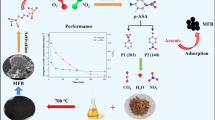
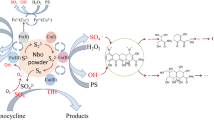
Persulfate (PS) activation technologies were of significant importance to the organic contaminant treatment. In this study, ascorbic acid (AA) was introduced to the traditional PS-activated process by using magnetite (Fe3O4) as the activator; herein, the degradation efficiency of sulfadimidine (SM2) was improved from 30 to 93% within 3 h, and the observed removal rate was about 8.0 times higher than that of the Fe3O4/PS system. These improvements were found to be induced by the added AA because it could reduce the surface Fe(III) to Fe(II) on Fe3O4 and thus facilitate the Fe(III)/Fe(II) cycle, which was conducive to producing reactive oxygen species (ROSs) in the oxidation process during PS activation. Meanwhile, AA could also promote the Fe(III)/Fe(II) cycle in the homogeneous solution, further advancing the PS decomposition for SM2 degradation. The ROS trapping experiments indicated that SM2 removal in the Fe3O4/PS/AA system was attributed to •OH and •SO4−, and •SO4− was the dominant ROS. Moreover, the reusability test experiment revealed that magnetite retained good activity after five cycles in the Fe3O4/AA/PS system. This study provides a promising PS activation technology for efficient organics contaminant treatment.










Similar content being viewed by others
Data availability
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
References
Abbas N, Shao GN, Imran SM, Haider MS, Kim HT (2016) Inexpensive synthesis of a high-performance Fe3O4-SiO2-TiO2 photocatalyst: magnetic recovery and reuse. Front Chem Sci Eng 10:405–416
Bao X, Qiang Z, Chang J, Ben W, Qu J (2014) Synthesis of carbon-coated magnetic nanocomposite (Fe3O4@C) and its application for sulfonamide antibiotics removal from water. J Environ Sci 26:962–969
Cao J, Lai L, Lai B, Yao G, Chen X, Song L (2019) Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: performance, intermediates, toxicity and mechanism. Chem Eng J 364:45–46
Chen Z, Geng S, Xiao J, Zhao F, Wan K, Wang Y, Tsiakaras P, Song S (2022) Understanding the selectivity trend of water and sulfate (SO42−) oxidation on metal oxides: on-site synthesis of persulfate, H2O2 for wastewater treatment. Chem Eng J 431:134332
Comninellis C, Kapalk A, Malat S, Parsons SA, Poulios I, Mantzavino D (2008) Advanced oxidation processes for water treatment: advances and trends for R&D. J Chem Techno Biot 83:769–776
Feng Z, Zhou B, Yuan R, Li H, He P, Wang F, Chen Z, Chen H (2022) Biochar derived from different crop straws as persulfate activator for the degradation of sulfadiazine: influence of biomass types and systemic cause analysis. Chem Eng J 440:135669
Forouzesh M, Ebadi A, Ghaeinejad-Meybodi A (2019) Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator. Sep Purif Technol 210:145–151
Fouad K, Alalm MG, Bassyouni M, Saleh MY (2020) A novel photocatalytic reactor for the extended reuse of W-TiO2 in the degradation of sulfamethazine. Chemosphere 257:127270
Furman OS, Teel A, Watts RJ (2010) Mechanism of base activation of persulfate. Environ Sci Technol 44:6423–6428
Gao F, Li Y, Xiang B (2018) Degradation of bisphenol A through transition metals activating persulfate process. Ecotoxicol Environ Safe 158:239–247
Giwa A, Yusuf BHA, Sambudi NS, Bilad MR, Adeyemi I, Chakraborty S, Curcio S (2021) Recent advances in advanced oxidation processes for removal of contaminants from water: a comprehensive review. Process Saf Environ Prot 146:220–256
Gui Q, Hu Y, Wang S, Zhang L (2022) Mechanism of synergistic pretreatment with ultrasound and ozone to improve gold and silver leaching percentage. Appl Surf Sci 576:151726
He F, Ma W, Zhong D, Yuan Y (2020) Degradation of chloramphenicol by α-FeOOH-activated two different double-oxidant systems with hydroxylamine assistance. Chemosphere 250:126150
Hou X, Huang X, Jia F, Ai Z, Zhao J, Zhang L (2017) Hydroxylamine promoted goethite surface Fenton degradation of organic pollutants. Environ Sci Technol 51:5118–5126
Hou X, Huang X, Li M, Zhang Y, Yuan S, Ai Z, Zhao J, Zhang L (2018) Fenton oxidation of organic contaminants with aquifer sediment activated by ascorbic acid. Chem Eng J 348:255–262
Hou X, Zhan G, Huang X, Wang N, Ai Z, Zhang L (2020) Persulfate activation induced by ascorbic acid for efficient organic pollutants oxidation. Chem Eng J 382:122355
Hu L, Zhang G, Wang Q, Wang X, Wang P (2019) Effect of microwave heating on persulfate activation for rapid degradation and mineralization of p-nitrophenol. ACS Sustain Chem Eng 7:11662–11671
Huang X, Hou X, Jia F, Song F, Zhao J, Zhang L (2017) Ascorbate-promoted surface iron cycle for efficient heterogeneous Fenton alachlor degradation with hematite nanocrystals. ACS Appl Mater Interfaces 9:8751–8758
Jin Z, Li Y, Dong H, Xiao S, Xiao J, Chu D, Hou X, Xiang S, Dong Q, Li L (2022) A comparative study on the activation of persulfate by mackinawite@biochar and pyrite@biochar for sulfamethazine degradation: the role of different natural iron-sulfur minerals doping. Chem Eng J 448:137620
Kuang H, He Z, Li M, Huang R, Zhang Y, Xu X, Wang L, Chen Y, Zhao S (2021) Enhancing co-catalysis of MoS2 for persulfate activation in Fe3+-based advanced oxidation processes via defect engineering. Chem Eng J 417:127987
Liu Y, Sheng X, Habib M, Wang P, Lu Z, Dong J, Sui Q, Lyu S (2023) FeS as excellent co-activator driving nano calcium peroxide oxidation for contaminants degradation: performance and mechanisms. Chemosphere 338:139559
Lominchar MA, Santos A, de Miguel E, Romero A (2018) Remediation of aged diesel contaminated soil by alkaline activated persulfate. Sci Total Environ 622–623:41–48
Luo H, Zeng Y, He D, Pan X (2021) Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: a review. Chem Eng J 407:127191
Meng F, Song M, Song B, Wei Y, Cao Q, Cao Y (2020) Enhanced degradation of Rhodamine B via α-Fe2O3 microspheres induced persulfate to generate reactive oxidizing species. Chemosphere 243:125322
Miklos DB, Remy C, Jekel M, Linden KG, Drewes JE, Hübner U (2018) Evaluation of advanced oxidation processes for water and wastewater treatment - a critical review. Water Res 139:118–131
Nikić J, Tubić A, Watson M, Maletić S, Šolić M, Majkić T, Agbaba J (2019) Arsenic removal from water by green synthesized magnetic nanoparticles. Water 11:2520
Oh WD, Veksha A, Chen X, Adnan R, Lim JW, Leong KH, Lim TT (2019) Catalytically active nitrogen-doped porous carbon derived from biowastes for organics removal via peroxymonosulfate activation. Chem Eng J 374:947–957
Shen W, Zhang J, Xiao M, Zhang X, Li J, Jiang W, Yan J, Qin Z, Zhang S, He W, He Y (2020) Ethylenediaminetetraacetic acid induces surface erosion of zero-valent iron for enhanced hexavalent chromium removal. Appl Surf Sci 525:146593
Sun H, Xie G, He D, Zhang L (2020) Ascorbic acid promoted magnetite Fenton degradation of alachlor: mechanistic insights and kinetic modeling. Appl Catal B-Environ 267:118383
Tan C, Gao N, Deng Y, Rong W, Zhou S, Lu N (2013) Degradation of antipyrine by heat activated persulfate. Sep Purif Technol 109:122–128
Wan Z, Hu J, Wang J (2016) Removal of sulfamethazine antibiotics using Ce-Fe-graphene nanocomposite as catalyst by Fenton-like process. J Environ Manage 182:284–291
Wu J, Wang B, Blaney L, Peng G, Chen P, Cui Y, Deng S, Wang Y, Huang J, Yu G (2019) Degradation of sulfamethazine by persulfate activated with organo-montmorillonite supported nano-zero valent iron. Chem Eng J 361:99–108
Yan H, Wang J, Zhang Y, Hu W (2016) Preparation and inhibition properties of molybdate intercalated ZnAlCe layered double hydroxide. J Alloys Compd 678:171–178
Yin R, Hu L, Xia D, Yang J, He C, Liao Y, Zhang Q, He J (2020) Hydroxylamine promoted Fe(III)/Fe(II) cycle on ilmenite surface to enhance persulfate catalytic activation and aqueous pharmaceutical ibuprofen degradation. Catal Today 358:294–302
Yu M, Mao X, He X, Zheng M, Zhang X, Su J, Xi B (2022) Efficient degradation of sulfamethazine in a silicified microscale zero-valent iron activated persulfate process. Appl Catal B-Environ 312:121418
Zeng X, Wang L, Zhang Y, Zhou S, Yu Z, Liu X, Chen C (2022) Enhanced removal of organic pollutants by ball-milled FeS/ZVI activated persulfate process: characterization, performance, and mechanisms. Surf Interfaces 29:101697
Zhang L, Liu B, Li J (2020) A novel synthesis method of mesoporous carbon loaded with Fe3O4 composite for effective adsorption and degradation of sulfamethazine. J Mol Liq 299:112096
Zhang H, Li L, Chen N, Ben H, Zhan G, Sun H, Li Q, Sun J, Zhang L (2022) Hydroxylamine enables rapid heterogeneous-homogeneous coupled Fenton sulfamethazine degradation on ferric phosphate. Appl Catal B-Environ 312:121410
Zhang H, Xie C, Chen L, Duan J, Li F, Liu W (2023) Different reaction mechanisms of SO4•− and •OH with organic compound interpreted at molecular orbital level in Co(II)/peroxymonosulfate catalytic activation system. Water Res 229:119392
Zhao Y, Sun C, Sun J, Zhou R (2015) Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep Purif Technol 142:182–188
Zhu F, Wu H, Zhang H, Komarneni S, Ma J (2022) Heterogeneous activation of persulfate by Bi2MoO6–CuS composite for efficient degradation of orange II under visible light. Chemosphere 293:133558
Funding
This work was supported by the Natural Science Foundation of Guangdong Province (2023A1515012134, 2022A1515010368), Characteristic Innovation Projects Guangdong Provincial Department of Education (Natural Science Category, 2021KQNCX084), and the Student Science and Technology Innovation Cultivating Program of Guangdong Province (pdjh2021b0457).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Xiaobing Wang, Meiting Zhi, Jingyi Li, Kunchuang Lin, Xueqin Lin, and Yue Hu. The first draft of the manuscript was written by Xiaobing Wang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhi, M., Li, J. et al. Ascorbic acid promoted sulfadimidine degradation in the magnetite-activated persulfate system by facilitating the Fe(III)/Fe(II) cycle. Environ Sci Pollut Res 31, 6481–6491 (2024). https://doi.org/10.1007/s11356-023-31566-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31566-6