Abstract
Organic UV filters (OUVFs), the active ingredient in sunscreens, are of environmental concern due to reported ecotoxicological effects in aquatic biota. Determining the environmental concentrations of these chemicals is essential for understanding their fate and potential environmental risk. Salting‐out assisted liquid–liquid extraction (SALLE) coupled with liquid-chromatography tandem mass spectrometry (LC–MS/MS) was developed for simultaneous extraction, separation, and quantification of seven OUVFs (2,4-dihydroxybenzophenone, 2,2′,4,4′-tetrahydroxybenzophenone, 4-methylbenzylidene camphor, butyl-methoxy-dibenzoyl methane, octocrylene, octyl methoxycinnamate, and oxybenzone). Method detection limits (MDLs) ranged from 11 to 45 ng/L and practical quantification limits (PQLs) from 33 to 135 ng/L. Method trueness, evaluated in terms of recovery, was 69–127%. Inter-day and intra-day variability was < 6% RSD. The coefficients of determination were > 0.97. The method was applied to river and seawater samples collected at 19 sites in and near Port Phillip Bay, Australia, and temporal variation in OUVF concentrations was studied at two sites. Concentrations of OUVF were detected at 10 sites; concentrations of individual OUVFs were 51–7968 ng/L, and the maximum total OUVF concentration detected at a site was 8431 ng/L. Recreational activity and water residence time at the site contributed to OUVF’s environmental presence and persistence. The benefits of the SALLE-LC–MS/MS method include its simple operation, good selectivity, precision over a wide linear range, and that obtained extracts can be directly injected into the LC–MS/MS, overall making it an attractive method for the determination of these OUVFs in environmental water matrices. To our knowledge, this is the first report of the occurrence of OUVFs in Port Phillip Bay, Australia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organic UV filters (OUVFs) are synthetic compounds that protect against damage from ultraviolet radiation (Ramos et al. 2015). They are the active ingredient in sunscreens and are incorporated in a wide range of personal care and manufactured products (e.g. shampoos, cosmetics, plastics, textiles, paints). Due to their wide use, they can enter freshwater and marine environments directly, for example when people engage in recreational activities or via industrial and wastewater discharge (Benedé et al. 2014; Tsui et al. 2014; Labille et al. 2020; O’Malley et al. 2020). Organic UV filters are becoming an important class of contaminants of emerging concern as they are increasingly detected in the environment. Toxicological data regarding OUVFs has shown harmful effects in aquatic species related to their endocrine-disrupting potential and genotoxic capabilities (Schlumpf et al. 2004; Downs et al. 2014, 2016; Wang et al. 2016; Ozaez et al. 2016; Carve et al. 2021). Consequently, the sale of sunscreens containing certain OUVFs, such as oxybenzone (BP-3) and octocrylene (OCT), have been restricted in some regions (Republic of Palau 2018; Hawaii 2018; Miller et al. 2021). Determining the environmental concentrations of these contaminants is essential for understanding their fate and potential risk to aquatic environments.
The physicochemical properties of OUVFs determine their fate in the environment and are an important consideration for optimization of analytical detection methods (Cadena-Aizaga et al. 2020). Typically, OUVFs contain single or multiple aromatic structures attached to hydrophobic groups (Ramos et al. 2016). Most are lipophilic, non-volatile, have a log Kow ≥ 4, and low to nil aqueous solubility. OUVFs have a limited absorption band spectrum; thus, sunscreens and other commodities often contain multiple OUVFs to provide protection against UVA (320–400 nm) and UVB (280–320 nm) (Ramos et al. 2016). Consequently, mixtures of OUVFs are expected to occur and have been detected at ng–mg/L levels in water samples from coastal and freshwater environments (Benedé et al. 2014; Tsui et al. 2014; Allinson et al. 2018; Labille et al. 2020; O’Malley et al. 2020). Oxybenzone (benzophenone-3; BP3) is the most frequently detected OUVF (Cadena-Aizaga et al. 2020); other more commonly observed OUVFs are 4-methylbenzylidene camphor (4-MBC), octyl methoxycinnamate (OMC), and octocrylene (OCT) (Cuccaro et al. 2022).
Determining the environmental concentrations of OUVFs is challenging due to the low concentrations and complex matrices typical of environmental water samples (Cadena-Aizaga et al. 2020). Accurate determination relies on high sensitivity of the analytical method and optimization of the sample preparation method (extraction, purification, and concentration). Extraction techniques more used include liquid–liquid extraction (LLE) (Jeon et al. 2006; Pintado-herrera et al. 2016), stir-bar sorptive extraction (Kawaguchi et al. 2006; Pintado-Herrera et al. 2013), single-drop microextraction (SDME) (Okanouchi et al. 2008) dispersive liquid microextraction (Tarazona et al. 2010), and solid-phase extraction (SPE) (Negreira et al. 2009; León et al. 2010; Kameda et al. 2011), with SPE being the most popular (Gago-Ferrero et al. 2013; Ramos et al. 2015). Disadvantages of some of these approaches may include large sample (~ 1 L) and solvent volume, lengthy extraction times, or the inadequacy of the method for the extraction of polar analytes (Cadena-Aizaga et al. 2020).
Salting-out assisted liquid–liquid extraction (SALLE) is based on LLE and has several advantages over other extraction methods. In SALLE, a salt is added to achieve the separation of aqueous phase from the partially miscible organic phase, and simultaneously, the target analytes are extracted into the separated organic phase (Valente et al. 2013; Wen et al. 2013). The organic phase can then be used directly for analysis. SALLE is a relatively simple method to perform and requires a small volume of samples and solvents compared to the other commonly used extraction techniques (e.g. SPE) (Razmara et al. 2011). SALLE has been successfully applied for the determination of a range of chemicals including pesticides and synthetic dyes in different types of matrices (Razmara et al. 2011; Valente et al. 2013; Wen et al. 2013; Gure et al. 2014).
In this study, SALLE was optimised for the extraction of seven OUVFs from environmental water samples. The target analytes were 2,4-dihydroxybenzophenone (BP-1), 2,2′,4,4′-tetrahydroxybenzophenone (BP-2), oxybenzone (BP-3), 4-methylbenzylidene camphor (4-MBC), butyl-methoxy-dibenzoylmethane (B-MDM), octyl methoxycinnamate (OMC), and octocrylene (OCT). The approach was validated by evaluating method detection limits (MDLs), practical quantification limits (PQLs), method trueness and inter-day precision, method linearity, and matrix recovery. The method was used to determine the concentrations of the target analytes in seawater and river water samples collected at 19 sites in and near Port Phillip Bay, Australia. To our knowledge, this is the first report of OUVF concentrations in Port Phillip Bay.
Materials and methods
Chemicals and standards
Analytical standards and the isotopically labelled analogue oxybenzone-(phenyl-13C6) were purchased from Novachem (Heidelberg West, VIC, Australia). The target analytes and their physical–chemical properties are shown in Table 1. Standards were purchased as solutions of 100 µg/mL in methanol (4-MBC, B-MDM, OMC) or acetonitrile (ACN; BP-3, OCT), except for BP-2, which was not dissolved in solvent, and BP-1 which was a 1000 µg/mL solution in 8:2 hexane:acetone (v/v). Linear calibration curves were constructed by gravimetric dilution of standard stock solutions in 70:30 ACN:ultrapure water (> 18Ω, Milli-Q, Millipore). All solvents and chemicals were of analytical grade. Formic acid, glacial acetic acid, and ammonium acetate (≥ 99.99%) were purchased from SigmaAldrich (Castle Hill, NSW, Australia). A combined standard solution (100 µg/L) and a surrogate stock solution (50 µg/L oxybenzone-(phenyl-13C6)) used for spiking were prepared in ACN (LC–MS grade, Honeywell, USA, and LiChrosolv hypergrade, Merck Millipore, Australia) and stored at − 20 °C. Sodium chloride (NaCl, ≥ 99.0%), sodium sulphate (Na2SO4, ≥ 99.0%), calcium chloride (CaCl, ≥ 99.0%), and magnesium chloride (MgSO4, ≥ 99.0%) were purchased from SigmaAldrich (Castle Hill, NSW, Australia) and Rowe Scientific (Dovetone, VIC, Australia). Quality controls (QCs), matrix spike (seawater spiked with OUVF standard and isotopically labelled surrogate), blanks spike (ultrapure water spiked with OUVF standard and isotopically labelled surrogate), and blank (ultrapure water) were prepared on the day of analysis and analysed alongside environmental samples. All QCs were extracted using the SALLE protocol described below.
Optimization of SALLE procedure
Each seawater sample (10 mL) spiked with combined standard solution was mixed with acidified ACN (0.5 M Formic acid) at a ratio of 1:1 (v/v) in analytically certified amber glass vials. Samples were vortexed for 30 s and then secured in a TCLP Rotary Agitator and rotated for 1 h at 30 ± 2 rpm at ambient temperature. To initiate the salting-out process, 4 or 8 g of either NaCl, Na2SO4, CaCl, or MgCl was added to each vial. Vials were vortexed for 1 min to achieve two distinct and well-separated phases. An aliquot from the ACN layer was filtered (0.22 µm cellulose filters, Terumo Australia pty ltd, Macquarie Park, NSW), and 500 µl was transferred to a LC vial to which 500 µl of acidified (0.5 M Formic acid) ultrapure water was added in preparation for chromatographic analysis. All the experiments were performed at room temperature (20 °C), and samples were protected from light to avoid possible photodegradation.
Instrumentation and chromatographic analysis
LC–MS/MS analysis was performed using the combined Shimadzu Nexera Z2 UHPLC and LCMS-8060 system equipped with an electrospray ionisation source and coupled with the Nexera X2 SIL-30ACMP Autosampler (Shimadzu). System control was with LabSolutions LCMS software (Shimadzu). Chromatographic separations were performed on a C18 column (Shim-pack XR-ODS, 3 mm I.D. × 30 mm, 1.62 µm, Shimadzu) with a guard column (Shim-pack XR-ODS, 3 mm I.D. × 30 mm, Shimadzu). Column temperature was 40 °C, and the injection volume was 40 µL. Mobile phases A and B were ACN, and a mixture of 5 mM ammonium acetate and 0.05% acetic acid in ultrapure water (v/v), respectively. The gradient was programmed as follows: 0.0–1.0 min, 10% A; 1.0–2.0 min, 50% A; 2.0–6.0 min, 100% A; 6.0–10.0 min, 100% A; and back to 10% in 1.0 min. The total time of analysis was 13.5 min. Oxybenzone-(phenyl-13C6) was used as a surrogate. Tandem mass spectrometer (MS/MS) conditions were optimised for each OUVF by varying MS/MS parameters using injections of analytical standard. General parameters were as follows: interface temperature 300 °C, DL temperature 250 °C, heat block temperature is 400 °C, drying gas flow is 10 L/min, heating gas flow 10 L/min, and nebulizing gas flow is 3 L/min. Electrospray ionisation in both negative and positive ion modes was performed in multiple reaction monitoring (MRM) conditions. The optimised MRM transitions are summarised in Table 2.
Method evaluation and quantitation limits
The SALLE combined with LC–MS/MS method was validated with respect to method linearity, detection limits (MDLs), practical quantification limits (PQLs), method trueness, and intra-day and inter-day precision. Method linearity was investigated over a concentration range of 0.04–1.5 ng/mL by plotting corresponding concentrations estimated from LC–MS/MS peak areas versus nominal OUVF concentrations and using least squares regression analysis in the R stats package (R Core Team 2020) in R Studio (v 2022.07.1, R v. 4.1.3). For each OUVF, MDLs, and PQLs were calculated using Eqs. 1 and 2, where t is the one-sided t distribution (t = 2.821), and I is the injected concentration (I = 200 pt).
Instrument inter-day and intra-day precision was assessed by calculating the relative standard deviation (%RSD) as in Eq. 3, where Xc is the mean surrogate-corrected value (n = 7).
Method trueness was assessed by calculating the percent recovery for spiked seawater and Milli-Q water samples as in Eq. 4, where Sc is the spiking concentration and Xc is the mean surrogate-corrected value (n = 2).
Application of SALLE-LC/MS to environmental samples
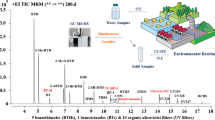
Environmental water samples were collected at 19 sites in and around Port Phillip Bay (PPB), Australia (n = 3 per site and sampling time) (Fig. 1). Four sites were rivers terminating in PPB (n = 12), 11 sites were beaches in PPB that are popular for recreational activities (n = 39), 3 sites were at Mornington Peninsula Ocean beaches (beach site n = 9, and rock pools n = 12), and 1 site was located near the centre of the entrance to PPB (Popes Eye, n = 3). To investigate temporal variation, samples were collected at two sites (Rye Bay Beach and Sorrento rockpool) in the early morning, midday, and late evening (n = 3 per site, per time point).
The sampling sites in and around Port Phillip Bay (PPB), Australia. The water types sampled were Rivers (Maribyrnong River, Mri, n = 3; Patterson River, Pri, n = 3; Werribee River, Wri, n = 3); Yarra River, Yri, n = 3), PPB beaches (Brighton, BBb, n = 3; Carrum, Cab, n = 3; Geelong Eastern, Geb, n = 3; Mount Martha, MMb, n = 3; Portsea Pier, PPb, n = 3; Quarantine Station, PNb, n = 3; Ricketts Point, RPb, n = 3; Rye Pier, RYb, n = 3 per time point, 9 samples in total; St Kilda, SKb, n = 3; Williamstown, WIb, n = 3; Williamstown Crystals, WCb, n = 3), Ocean Beaches (Sorrento Ocean beach, SBb, n = 3)), and rockpools (Sorrento rockpool, SBrp, n = 3 per time point, 9 samples in total; Bridgewater Bay, BBrp, n = 3), and one site that was located toward the center of the entry to Port Phillip Bay at Popes Eye (POE, n = 3)
Samples were collected by opening an analytically certified amber glass vial (40 mL) under the water’s surface, allowing the bottle to passively fill, and replacing the lid while the vial was still submerged. Samples were collected 10 to 20 cm under the water’s surface and 3 to 10 m from shore. Due to the risk of sample contamination, the use of personal care products containing OUVFs was avoided before and during the sampling. At each site, the number of people engaged in recreational activity was estimated by counting the number of beachgoers in the water. Climate data on daily solar exposure (MJ m−2) and weather parameters (air and water temperature, wind speed and direction, tidal movement) were measured during sampling or compiled from publicly available data on the Australian Bureau of Meteorology website (2022) (Supplementary file 1, Table S1). Samples were stored in the dark on ice during collection and transported to ADE Consulting laboratory, Melbourne, where they remained in storage in the dark at 4 °C until analysis.
Results and discussion
Optimization of SALLE
Sample preparation is integral to the sensitivity and trueness of an analytical method (Płotka-Wasylka et al. 2021). In SALLE, important factors that influence performance are the selected salt and salt concentration and the selected organic solvent (Camino-Sánchez et al. 2011). The salt’s anion is considered responsible for efficient phase separation, and different salt types and salt concentrations can induce varying grades of phase separation due to differing ionic strengths of the aqueous donor phase, which affects the analytes solubility in the aqueous phase and consequent transfer to the organic phase (Asensio-ramos et al. 2011; Wen et al. 2013; Benedé et al. 2014; Gure et al. 2014). The salting-out effect can improve the extraction of polar analytes from the aqueous phase, particularly for extraction of polar compounds (log Kow < 4) with hydroxyl groups (e.g. BP-3) (Pintado-Herrera et al. 2014; Vila et al. 2016a).
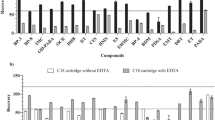
Four different salts (NaCl, Na2SO4, CaCl, MgCl) at two different amounts (4 or 8 g/sample) were tested in this study (Fig. 2). All salts induced phase separation at the chosen concentrations. In most cases, increasing the salt amount from 4 to 8 g provided only a small change in recovery (Fig. 2). For subsequent experiments, Na2SO4 was chosen since it is relatively non-toxic and produced good phase separation and recovery of target analytes (Table 4). Furthermore, SO42− is ranked above Cl− in the Hofmeister series, thus expected to be more efficient at phase separation than Cl− (Alshishani et al. 2017). For Na2SO4, the difference in analyte recovery between an addition of 4 g or 8 g of salt was < 5% for BP-2, BP-3, and B-MDM, and < 7% for BP-1 and OMC. For 4-MBC, addition of 4 g Na2SO4 increased mean recoveries from 55.1 to 92.1%, whereas for OCT, addition of 8 g Na2SO4 increased mean recoveries from 76.8 to 94.9%. Poor phase separation was observed from an amount of Na2SO4 < 2.5 g.
The effect of different salts (NaCl, Na2SO4, CaCl, and MgCl) and two salt amounts (4 and 8 g) on extraction efficiency (mean ± standard error) of seven organic UV filters from seawater using SALLE. Extraction conditions: 10 mL sample volume, 10 mL acetonitrile. Target analytes were 4-methylbenzylidene camphor (4-MBC), butyl-methoxy-dibenzoylmethane (B-MDM), 2,4-dihydroxybenzophenone (BP-1), 2,2′,4,4′-tetrahydroxybenzophenone (BP-2), oxybenzone (BP-3), octocrylene (OCT), and octyl methoxycinnamate (OMC)
Acetonitrile was chosen as the organic solvent due to its attractive properties for use in SALLE, specifically its highly polarity, miscibility in water, relatively low toxicity, and that it can be directly injected into LC system (Gure et al. 2014; Sereshti et al. 2014). Solvent volume is an important consideration that can affect SALLE efficiency: too small a volume results in the phase boundary between acetonitrile and sample being too difficult to distinguish, whereas too large a volume results in over dilution of analytes (Sereshti et al. 2014). A volume of 10 mL was found to be suitable for the application and used in subsequent experiments.
Analytical performance
Separation, identification, and quantification of OUVFs were performed using liquid-chromatography tandem–mass spectrometry (LC–MS/MS). Analytes separated along the LC column within 13 min. The addition of formic acid increased the sharpness and resolution of chromatic peaks. Furthermore, addition of formic acid is critical to disrupting compound–protein binding which may occur in environmental samples (Picot Groz et al. 2014). The behaviour of all compounds was linear and exhibited a direct proportional relationship between the amount of each analyte and the chromatographic response. The coefficients of determination (R2) were above 0.97 (Table 3). Inter-day and intra-day precision, expressed in terms of relative standard deviation (%RSD), was < 6% RSD for all compounds (Table 3). Method detection limits (MDLs) ranged from 11 to 45 ng/L and practical quantification limits (PQLs) from 33 to 135 ng/L (Table 3). Method trueness was assessed in a recovery study carried out with seawater and Milli-Q water samples at two concentrations (Table 4). For Milli-Q samples, average recovery values for the two spike concentrations were 91–109%. Seawater samples also showed good recovery, with average recovery values for the two spike concentrations being 69–127%. A representative chromatogram for an environmental sample is shown in Fig. 3 and for the OUVF combined standards solution is shown in Fig. S1 (Supplementary file 1). A comparison with other methods for determining OUVF concentrations in seawater published in the last 10 years is provided in Table 5. There were some differences among methods regarding the number of targeted OUVFs, analytical performance, and sample volume; however, overall the analytical performance of the methods was similar. All studies had recoveries > 63%. LOD were at trace concentrations in all cases (ng/L). Most methods required a sample volume of ≥ 100 mL, but solid phase microextraction techniques required a smaller sample volume of 10–100 mL. Similar to microextraction techniques, our presently described SALLE method has the advantage of requiring a small sample volume of 10 mL.
Representative chromatogram for an environmental sample showing organic UV filter mixture: oxybenzone (BP-3), octocrylene (OCT), 4-methylbenzylidene camphor (4-MBC), and butyl-methoxy-dibenzoylmethane (B-MDM), 2,4-dihydroxybenzophenone (BP-1), and octyl methoxycinnamate (asterisk indicates below MDL)
Other important considerations are if derivatization and concentration steps are required, extraction time and ‘greenness’ of solvents used. In the compiled studies, the analytical instrument most often used is GC (Table 5). In our method, we employed LC since it has the advantage not requiring a derivatization step, which is required to increase chromatographic efficiency when using GC due to the high polarity of OUVFs (Jeon et al. 2006; Kotnik et al. 2014; Vila et al. 2017). The extraction technique most often used in the compiled studies and in earlier studies was SPE (Jeon et al. 2006; Bratkovics and Sapozhnikova 2011; Cadena-Aizaga et al. 2020). Compared to the developed SALLE method, SPE can require a large volume of sample and solvents and may require time consuming concentration and evaporation steps. SALLE was used in one other study compiled in Table 5 (Labille et al. 2020), and targeted four OUVFS, used 50 mL sample volume, 200 g/L Na2SO4, 5 mL methyl tertiary-butyl ether and required additional steps for concentration and evaporation. In contrast, our work used a smaller sample volume and did not require additional concentration and evaporation steps to achieve trace-level environmentally relevant MDLs and PQLs for the targeted OUVFs. Concentration by evaporation can increase sample processing time, reduce method robust, and can limit a methods potential for industrial scale processing. In summary, the developed SALLE method has advantages when compared with other methods regrading simplicity of operation, greenness of analytical method, and has a satisfactory performance suitable for its application.
Environmental sample analysis
The optimised method was applied to the analysis of surface water samples collected at 19 sites in and near PPB, Australia. All environmental samples were spiked with surrogate oxybenzone-(phenyl-13C6) (100 ng/L), and surrogate recoveries were 77–151%. The sites represented a range of waterbody types including rivers near PPB, PPB beaches, the centre of the entry to PPB, and ocean beaches and rockpools near PPB (Fig. 1). Of the 7 target OUVFs, 4-MBC, B-MDM, OCT, and BP-3 were detected in one or more water samples collected at 10 of the 19 study sites (Table 6). Maximum total OUVF concentrations detected at sites ranged from 74 to 8597 ng/L per site, which were observed at Quarantine Station Beach and Williamstown Beach, respectively. The most widely detected OUVFs were 4-MBC (9 sites) and OCT (7 sites). Both BP-3 and B-MDM were detected at 4 sites. The most frequently detected OUVF was 4-MBC, which was detected in 37% of the water samples (29 samples), followed by OCT (18%, 14 samples), BP-3 (15%, 12 samples), and B-MDM (10%, 8 samples). B-MDM was detected at the highest maximum concentration (7968 ng/L), which was observed at Williamstown Beach, and was at least four-fold higher than the maximum concentration detected for 4-MBC (1643 ng/L, Sorrento Ocean Beach Rockpool), BP-3 (473 ng/L, Ricketts Point Beach), and OCT (99 ng/L, Sorrento Ocean Beach Rockpool).
The level of recreational activity was characterised for each site by counting the number of beachgoers at the site at the time of sampling since recreational activity has previously been shown as a significant source of OUVFs to marine environments (Labille et al. 2020). Comparing OUVF concentrations observed at sites characterised by high level of recreational activity (e.g. Rye Bay Beach, Carrum Beach, and Williamstown beach) with sites characterised by a low level of recreational activity (e.g. Quarantine Station Beach, St Kilda Beach, and Portsea Bay Beach) clearly showed that people engaged in recreational activities are associated with significant introduction of OUVFs to PPB (Table 6). Total maximum OUVF concentrations observed at sites characterised by a high level of recreational activity were 119–8597 ng/L, whereas total maximum OUVF concentrations observed at sites characterised by a low level of recreational activity were 74–108 ng/L. In addition, the profiles of OUVFs in surface water demonstrated site-specific differences (Table 6), possibly reflecting differences in the sunscreen products used by people engaged in recreational activities, the photostability of the OUVFs in seawater, and dilution of OUVFs by vertical and horizontal transport in the water column (Labille et al. 2020).
Three rivers were sampled during an ebbing tide to estimate the contribution that wastewater treatment plant effluent and urban runoff may have on concentrations of OUVFs occurring in PPB. In our study, OUVFs were not detected at river sites, which is interesting since common OUVF have been previously detected in PPB estuaries (Allinson et al. 2018). Allinson et al. (2018) reported concentrations of 4-MBC, OMC, and OCT up to 642 ng/L, 640 ng/L, and 109 ng/L, respectively, and the average OUVF concentrations across sites were 7.4 ng/L. Differences between our study and Allinson et al. (2018) with regards to sampling time and location may have resulted in OUVFs not being detected in our study since OUVFs can be subject to photodegradation and dilution by transport in the environment.
Temporal variation in total OUVF concentration was studied at two sites, Rye Bay Beach, and Sorrento Ocean Beach rockpool (Fig. 1). Samples were collected in the early morning (low recreational activity), at midday (high recreational activity), and in the early evening (low recreational activity). Sampling times coincided with different tidal stages (supplementary material, Table S1). The geomorphology of the sites differed; Sorrento Ocean Beach rockpool represents a closed system that is flushed at hightide, whereas Rye Bay Beach is a sandy embayment within PPB subject to continual tidal movements and circulating Bay currents. At both sites, the highest total OUVF concentration was observed at midday during peak recreational activity and was 795 ng/L at Rye Bay Beach and 4716 ng/L at Sorrento Ocean Beach rockpool. In the evening, OUVFs were observed at both sites; however, the total OUVF concentrations were lower than at midday and were 536 ng/L and 516 ng/L at Sorrento Ocean Beach rockpool and Rye Ocean Beach, respectively. In addition, OUVF profiles at the sites differed between midday and evening. At both sites, B-MDM, OCT, BP-3, and 4-MBC were detected at midday, but in the evening, only 4-MBC was detected at Rye Ocean Beach, and OCT, BP-3, and 4-MBC were detected in the evening at Sorrento Ocean Beach rockpool.
Unexpectedly, OCT was detected at Popes Eye (80 ng/L, no recreational activity) and Quarantine Station Beach (74 ng/L, low level of recreational activity) (Table 6). Both sites are close the entrance to PPB and are subjected to high tidal movement of water caused by the narrow entry to the bay. In similar studies in marine environments, detected OCT concentrations have ranged from 75 ng/L to 171 µg/L (Tsui et al. 2014; Vila et al. 2016b). Unlike other OUVFs included in this study (e.g. B-MDM and BP-3), which were not detected at these sites, OCT is photostable and may be more likely transported vertically and horizontally in the waterbody (Santos et al. 2012; Manasfi et al. 2017; Labille et al. 2020).
The concentration values detected at beaches are similar to those reported in many other studies in coastal areas. For instance, Labille et al. (2020) surveyed Mediterranean beaches and found that OCT and B-MDM were most frequently detected with concentrations ranging from 75–425 ng/L to 10–350 ng/L, respectively, whereas BP-3 and OMC were detected less often with concentrations ranging from 50–75 mg/L to 2.6–8.8 ng/L, respectively. In coastal areas, OUVFs are typically detected at trace levels. For some OUVFs, however, higher maximum concentrations have been observed, for example BP-3 has been detected at 1.395 mg/L (Downs et al. 2016), OCT at 79 µg/L (Vila et al. 2017), and B-MDM at 72 µg/L (Vila et al. 2016a).
The differences in OUVF concentrations and profiles observed among sites in our study show that OUVF input to PPB is due to recreational activities and that the OUVF input is pulsed based on the activity level of beachgoers. This was particularly evident in the temporal study at Sorrento Ocean Beach rockpool. The site was sampled in the early morning (low recreational activity, no OUVFs detected), at midday before the pool was reached by the flooding tide, and in the evening after the pool had been flushed by the high tide with similar OUVF profiles observed at both times, but a higher total concentration observed at midday corresponding to peak recreational activity. Furthermore, the temporal study at Rye Bay Beach showed how OUVF environmental concentrations are reduced by dilution occurring due to transport in the water column and/or waterbody influenced by currents and site geomorphology.
Presently, there are no marine water quality guideline values for the target OUVFs in Australia (ANZECC & ARMCANZ 2000, 2018). Predicted no-effect concentrations (PNECs), which are important indicators of ecological risk, for the detected OUVFs have been reported as ≥ 40 ng/L 4-MBC, ≥ 100 ng/L OCT, and ≥ 10 ng/L BP-3 (Carve et al. 2021; Miller et al. 2021), but for B-MDM, no PNECs are available due to insufficient ecotoxicological data being available for their calculation. In this context, PNECs were exceeded at 9 sites for 4-MBC and 4 sites for BP-3. Furthermore, it is important to consider that OUVF concentrations can be higher in biota than observed in water samples due to bioconcentration (Cadena-Aizaga et al. 2022), and that the toxic effects of OUVF mixtures can occur at concentrations lower than observed for a single chemical (Escher and Hermens 2002). To this end, of the four OUVFs detected in this study, two or more were detected in 15 water samples, and in particular, OCT’s high log Kow (> 6) indicates its potential for bioaccumulation (Cadena-Aizaga et al. 2022). Results from this study indicate that the potential risk posed by 4-MBC, OCT, and BP-3 to Port Philip Bay aquatic ecosystems are appreciable, and further assessments of their occurrence in PPB and associated biota are paramount to evaluating their ecological risk.
Conclusion
A sensitive analytical method based on salting-out assisted liquid–liquid extraction (SALLE) and LC–MS/MS method has been developed. The method enables the determination of seven OUVFs at trace level in environmental water samples with good trueness and precision. The method is appropriate for analysis of target compounds at trace concentrations with low relative standard deviation (< 6%) and limits of detection (MDLs: 11 to 45 ng/L and PQLs: 33 to 135 ng/L). The proposed method is simple and efficient, and the protocol uses minimal amounts of organic solvents (10 mL/sample) and time (< 1.5 h/sample).
To our knowledge, this is the first report of the occurrence of the target OUVFs in the temperate environments in and near Port Phillip Bay, Australia. Results indicate that 4-MBC, BP-3, B-MDM, and OCT are detectable in the coastal surface water of Port Phillip Bay during summer and may pose an ecological risk to PPB. The OUVF B-MDM was detected at the highest concentrations, and 4-MBC was detected most widely. Williamstown Beach ranked the highest in terms of the total OUVF concentration present at a site. The concentrations of OUVFs detected at sites reflected the level of recreational activity observed at the time of sampling.
The temporal changes in OUVF concentration reflected patterns of recreational activities and localised water movement, influenced by tidal cycles, currents, and site geomorphology. Results suggest that OUVF input to the PPB marine environments is pulsed due to the activity of beachgoers, and that the persistence of OUVFs is influenced by water residence time at the site and OUVF stability in the environment. These data presented are essential to evaluating the potential risk posed by OUVFs to the Port Philp Bay marine environment.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Allinson M, Kameda Y, Kimura K, Allinson G (2018) Occurrence and assessment of the risk of ultraviolet filters and light stabilizers in Victorian estuaries. Environ Sci Pollut Res 25:12022–12033. https://doi.org/10.1007/s11356-018-1386-7
Alshishani AA, Saad B, Semail NF et al (2017) Salting-out assisted liquid-liquid extraction method coupled to gas chromatography for the simultaneous determination of thujones and pulegone in beverages. Int J Food Prop 20:S2776–S2785. https://doi.org/10.1080/10942912.2017.1373665
ANZECC & ARMCANZ (2000) Australian and New Zealand guidelines for fresh and marine water quality. Canberra, Australia
ANZECC & ARMCANZ (2018) Australian and New Zealand guidelines for fresh and marine water quality. https://www.waterquality.gov.au/anz-guidelines. Accessed 23 Mar 2023
Asensio-ramos M, Ravelo-pérez LM, González-curbelo MÁ, Hernández-borges J (2011) Liquid phase microextraction applications in food analysis. J Chromatogr A 1218:7415–7437. https://doi.org/10.1016/j.chroma.2011.05.096
Australian Bureau of Meteorology (2022) http://www.bom.gov.au/. Accessed Feb 2022
Benedé JL, Chisvert A, Salvador A et al (2014) Determination of UV filters in both soluble and particulate fractions of seawaters by dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. Anal Chim Acta 812:50–58. https://doi.org/10.1016/j.aca.2013.12.033
Bratkovics S, Sapozhnikova Y (2011) Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Anal Methods 3:2943–2950. https://doi.org/10.1039/c1ay05390f
Cadena-Aizaga MI, Montesdeoca-Esponda S, Torres-Padrón ME et al (2020) Organic UV filters in marine environments: an update of analytical methodologies, occurrence and distribution. Trends Environ Anal Chem 25:e00079. https://doi.org/10.1016/j.teac.2019.e00079
Cadena-Aizaga MI, Montesdeoca-Esponda S, Santana-Del Pino A et al (2022) Assessment of anthropogenic pollution by UV filters using macrophytes as bioindicators. Sci Total Environ 832:155012. https://doi.org/10.1016/j.scitotenv.2022.155012
Camino-Sánchez FJ, Zafra-Gómez A, Pérez-Trujillo JP et al (2011) Validation of a GC–MS/MS method for simultaneous determination of 86 persistent organic pollutants in marine sediments by pressurized liquid extraction followed by stir bar sorptive extraction. Chemosphere 84:869–881. https://doi.org/10.1016/j.chemosphere.2011.06.019
Carve M, Nugegoda D, Allinson G, Shimeta J (2021) A systematic review and ecological risk assessment for organic ultraviolet filters in aquatic environments. Environ Pollut 268:115894. https://doi.org/10.1016/j.envpol.2020.115894
Chisvert A, Benedé JL, Anderson JL et al (2017) Introducing a new and rapid microextraction approach based on magnetic ionic liquids: stir bar dispersive liquid microextraction. Anal Chim Acta 983:130–140. https://doi.org/10.1016/j.aca.2017.06.024
Cuccaro A, Freitas R, De Marchi L et al (2022) UV-filters in marine environments: a review of research trends, meta-analysis, and ecotoxicological impacts of 4-methylbenzylidene-camphor and benzophenone-3 on marine invertebrate communities. Environ Sci Pollut Res 29:64370–64391. https://doi.org/10.1007/s11356-022-21913-4
Downs CA, Kramarsky-Winter E, Fauth JE et al (2014) Toxicological effects of the sunscreen UV filter, benzophenone-2, on planulae and in vitro cells of the coral, Stylophora pistillata. Ecotoxicology 23:175–191. https://doi.org/10.1007/s10646-013-1161-y
Downs CA, Kramarsky-Winter E, Segal R et al (2016) Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol 70:265–288. https://doi.org/10.1007/s00244-015-0227-7
Escher BI, Hermens JLM (2002) Modes of action in ecotoxicology: their role in body burdens, species sensitivity, QSARs, and mixture effects. Environ Sci Technol 36:4201–4217. https://doi.org/10.1021/es015848h
Gago-Ferrero P, Diaz-Cruz MS, Barceló D (2013) Liquid chromatography-tandem mass spectrometry for the multi-residue analysis of organic UV filters and their transformation products in the aquatic environment. Anal Methods 5:355–366. https://doi.org/10.1039/C2AY26115D
Gure A, Lara FJ, Moreno-González D et al (2014) Salting-out assisted liquid-liquid extraction combined with capillary HPLC for the determination of sulfonylurea herbicides in environmental water and banana juice samples. Talanta 127:51–58. https://doi.org/10.1016/j.talanta.2014.03.070
Hawaii (2018) SB2571. https://www.capitol.hawaii.gov/sessions/session2018/bills/sb2571_cd1_.htm. Accessed 20 Mar 2023
Jeon HK, Chung Y, Ryu JC (2006) Simultaneous determination of benzophenone-type UV filters in water and soil by gas chromatography-mass spectrometry. J Chromatogr A 1131:192–202. https://doi.org/10.1016/j.chroma.2006.07.036
Kameda Y, Kimura K, Miyazaki M (2011) Occurrence and profiles of organic sun-blocking agents in surface waters and sediments in Japanese rivers and lakes. Environ Pollut 159:1570–1576. https://doi.org/10.1016/j.envpol.2011.02.055
Kawaguchi M, Ito R, Endo N et al (2006) Stir bar sorptive extraction and thermal desorption-gas chromatography-mass spectrometry for trace analysis of benzophenone and its derivatives in water sample. Anal Chim Acta 557:272–277. https://doi.org/10.1016/j.aca.2005.08.087
Kotnik K, Kosjek T, Krajnc U, Heath E (2014) Trace analysis of benzophenone-derived compounds in surface waters and sediments using solid-phase extraction and microwave-assisted extraction followed by gas chromatography-mass spectrometry. Anal Bioanal Chem 406:3179–3190. https://doi.org/10.1007/s00216-014-7749-0
Kung TA, Lee SH, Yang TC, Wang WH (2018) Survey of selected personal care products in surface water of coral reefs in Kenting National Park. Taiwan Sci Total Environ 635:1302–1307. https://doi.org/10.1016/j.scitotenv.2018.04.115
Labille J, Slomberg D, Catalano R, et al (2020) Assessing UV filter inputs into beach waters during recreational activity: a field study of three French Mediterranean beaches from consumer survey to water analysis. Sci Total Environ 706: https://doi.org/10.1016/j.scitotenv.2019.136010
León Z, Chisvert A, Tarazona I, Salvador A (2010) Solid-phase extraction liquid chromatography-tandem mass spectrometry analytical method for the determination of 2-hydroxy-4-methoxybenzophenone and its metabolites in both human urine and semen. Anal Bioanal Chem 398:831–843. https://doi.org/10.1007/s00216-010-3947-6
Li AJ, Sang Z, Chow C-HH et al (2017) Environmental behavior of 12 UV filters and photocatalytic profile of ethyl-4-aminobenzoate. J Hazard Mater 337:115–125. https://doi.org/10.1016/j.jhazmat.2017.04.067
Manasfi T, Coulomb B, Ravier S, Boudenne J-L (2017) Degradation of organic UV filters in chlorinated seawater swimming pools: transformation pathways and bromoform formation. Environ Sci Technol 51:13580–13591. https://doi.org/10.1021/acs.est.7b02624
Miller IB, Pawlowski S, Kellermann MY, et al (2021) Toxic effects of UV filters from sunscreens on coral reefs revisited: regulatory aspects for “reef safe” products. Environ Sci Eur 33:. https://doi.org/10.1186/s12302-021-00515-w
Negreira N, Rodríguez I, Ramil M et al (2009) Solid-phase extraction followed by liquid chromatography-tandem mass spectrometry for the determination of hydroxylated benzophenone UV absorbers in environmental water samples. Anal Chim Acta 654:162–170. https://doi.org/10.1016/j.aca.2009.09.033
O’Malley E, O’Brien JW, Verhagen R, Mueller JF (2020) Annual release of selected UV filters via effluent from wastewater treatment plants in Australia. Chemosphere 247: https://doi.org/10.1016/j.chemosphere.2020.125887
Okanouchi N, Honda H, Ito R et al (2008) Determination of benzophenones in river-water samples using drop-based liquid phase microextraction coupled with gas chromatography/mass spectrometry. Anal Sci 24:627–630. https://doi.org/10.2116/analsci.24.627
Ozaez I, Aquilino M, Morcillo G, Martinez-Guitarte J-L (2016) UV filters induce transcriptional changes of different hormonal receptors in Chironomus riparius embryos and larvae. Environ Pollut 214:239–247. https://doi.org/10.1016/j.envpol.2016.04.023
Picot Groz M, Martinez Bueno MJ, Rosain D et al (2014) Detection of emerging contaminants (UV filters, UV stabilizers and musks) in marine mussels from Portuguese coast by QuEChERS extraction and GC-MS/MS. Sci Total Environ 493:162–169. https://doi.org/10.1016/j.scitotenv.2014.05.062
Pintado-Herrera MG, González-Mazo E, Lara-Martín PA (2013) Environmentally friendly analysis of emerging contaminants by pressurized hot water extraction-stir bar sorptive extraction-derivatization and gas chromatography-mass spectrometry. Anal Bioanal Chem 405:401–411. https://doi.org/10.1007/s00216-012-6453-1
Pintado-Herrera MG, González-Mazo E, Lara-Martín PA (2014) Atmospheric pressure gas chromatography-time-of-flight-mass spectrometry (APGC-ToF-MS) for the determination of regulated and emerging contaminants in aqueous samples after stir bar sorptive extraction (SBSE). Anal Chim Acta 851:1–13. https://doi.org/10.1016/j.aca.2014.05.030
Pintado-Herrera MG, González-Mazo E, Lara-Martín PA (2016) In-cell clean-up pressurized liquid extraction and gas chromatography–tandem mass spectrometry determination of hydrophobic persistent and emerging organic pollutants in coastal sediments. J Chromatogr A 1429:107–118. https://doi.org/10.1016/j.chroma.2015.12.040
Płotka-Wasylka J, Mohamed HM, Kurowska-Susdorf A et al (2021) Green analytical chemistry as an integral part of sustainable education development. Curr Opin Green Sustain Chem 31. https://doi.org/10.1016/j.cogsc.2021.100508
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Ramos S, Homem V, Alves A, Santos L (2015) Advances in analytical methods and occurrence of organic UV-filters in the environment-a review. Sci Total Environ 526:278–311. https://doi.org/10.1016/j.scitotenv.2015.04.055
Ramos S, Homem V, Alves A, Santos L (2016) A review of organic UV-filters in wastewater treatment plants. Environ Int 86:24–44. https://doi.org/10.1016/j.envint.2015.10.004
Razmara RS, Daneshfar A, Sahrai R (2011) Determination of methylene blue and sunset yellow in wastewater and food samples using salting-out assisted liquid-liquid extraction. J Ind Eng Chem 17:533–536. https://doi.org/10.1016/j.jiec.2010.10.028
Republic of Palau (2018) Senate Bill No. 10–135, SD1, HD1 (The Responsible Tourism Education Act of 2018). https://www.palaugov.pw/wp-content/uploads/2018/10/RPPL-No.-10-30-re.-The-Responsible-Tourism-Education-Act-of-2018.pdf. Accessed 22 Mar 2023
Sanchez Rodriguez A, Rodrigo Sanz M, Betancort Rodriguez JR et al (2015) Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands). An approach to environmental risk assessment. Chemosphere 131:85–90. https://doi.org/10.1016/j.chemosphere.2015.02.054
Sankoda K, Murata K, Tanihata M et al (2015) Seasonal and diurnal variation of organic ultraviolet filters from personal care products used along the Japanese coast. Arch Environ Contam Toxicol 68:217–224. https://doi.org/10.1007/s00244-014-0106-7
Santos AJM, Miranda MS, Esteves da Silva JCG (2012) The degradation products of UV filters in aqueous and chlorinated aqueous solutions. Water Res 46:3167–3176. https://doi.org/10.1016/j.watres.2012.03.057
Schlumpf M, Schmid P, Durrer S et al (2004) Endocrine activity and developmental toxicity of cosmetic UV filters-an update. Toxicology 205:113–122. https://doi.org/10.1016/j.tox.2004.06.043
Sereshti H, Khosraviani M, Sadegh Amini-Fazl M (2014) Miniaturized salting-out liquid-liquid extraction in a coupled-syringe system combined with HPLC-UV for extraction and determination of sulfanilamide. Talanta 121:199–204. https://doi.org/10.1016/j.talanta.2014.01.005
Tarazona I, Chisvert A, León Z, Salvador A (2010) Determination of hydroxylated benzophenone UV filters in sea water samples by dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. J Chromatogr A 1217:4771–4778. https://doi.org/10.1016/j.chroma.2010.05.047
Tsui MMP, Leung HW, Wai T-CC et al (2014) Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res 67:55–65. https://doi.org/10.1016/j.watres.2014.09.013
Tsui MPP, Chen L, He T et al (2019) Organic ultraviolet (UV)filters in the South China sea coastal region: Environmental occurrence, toxicological effects and risk assessment. Ecotoxicol Environ Saf 181:26–33. https://doi.org/10.1016/j.ecoenv.2019.05.075
Valente IM, Gonçalves LM, Rodrigues JA (2013) Another glimpse over the salting-out assisted liquid-liquid extraction in acetonitrile/water mixtures. J Chromatogr A 1308:58–62. https://doi.org/10.1016/j.chroma.2013.08.014
Vila M, Celeiro M, Lamas JP et al (2016a) Determination of fourteen UV filters in bathing water by headspace solid-phase microextraction and gas chromatography-tandem mass spectrometry. Anal Methods 8:7069–7079. https://doi.org/10.1039/c6ay01787h
Vila M, Pablo Lamas J, Garcia-Jares C et al (2016b) Ultrasound-assisted emulsification microextraction followed by gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry for the analysis of UV filters in water. Microchem J 124:530–539. https://doi.org/10.1016/j.microc.2015.09.023
Vila M, Celeiro M, Pablo Lamas J et al (2017) Simultaneous in-vial acetylation solid-phase microextraction followed by gas chromatography tandem mass spectrometry for the analysis of multiclass organic UV filters in water. J Hazard Mater 323:45–55. https://doi.org/10.1016/j.jhazmat.2016.06.056
Wang J, Pan L, Wu S et al (2016) Recent advances on endocrine disrupting effects of UV filters. Int J Environ Res Public Health 13:1–11. https://doi.org/10.3390/ijerph13080782
Wen Y, Li J, Yang F et al (2013) Salting-out assisted liquid-liquid extraction with the aid of experimental design for determination of benzimidazole fungicides in high salinity samples by high-performance liquid chromatography. Talanta 106:119–126. https://doi.org/10.1016/j.talanta.2012.12.011
Acknowledgements
The authors would like to acknowledge the valuable contributions and support provided by Prof. Dayanthi Nugegoda and Dr. Timothy Coggan for various technical aspects of this project.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors. Megan Carve receives an Australian Government Research Training Program Scholarship through RMIT University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Megan Carve and Navneet Singh. The first draft of the manuscript was written by Megan Carve, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carve, M., Singh, N., Askeland, M. et al. Salting-out assisted liquid–liquid extraction combined with LC–MS/MS for the simultaneous determination of seven organic UV filters in environmental water samples: method development and application. Environ Sci Pollut Res 30, 104870–104885 (2023). https://doi.org/10.1007/s11356-023-29646-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29646-8