Abstract
Seagrasses harbour different and rich epiphytic bacterial communities. These microbes may establish intimate and symbiotic relationships with the seagrass plants and change according to host species, environmental conditions, and/or ecophysiological status of their seagrass host. Although Posidonia oceanica is one of the most studied seagrasses in the world, and bacteria associated with seagrasses have been studied for over a decade, P. oceanica’s microbiome remains hitherto little explored. Here, we applied 16S rRNA amplicon sequencing to explore the microbiome associated with the leaves of P. oceanica growing in two geomorphologically different meadows (e.g. depth, substrate, and turbidity) within the Limassol Bay (Cyprus). The morphometric (leaf area, meadow density) and biochemical (pigments, total phenols) descriptors highlighted the healthy conditions of both meadows. The leaf-associated bacterial communities showed similar structure and composition in the two sites; core microbiota members were dominated by bacteria belonging to the Thalassospiraceae, Microtrichaceae, Enterobacteriaceae, Saprospiraceae, and Hyphomonadaceae families. This analogy, even under different geomorphological conditions, suggest that in the absence of disturbances, P. oceanica maintains characteristic-associated bacterial communities. This study provides a baseline for the knowledge of the P. oceanica microbiome and further supports its use as a putative seagrass descriptor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posidonia oceanica is a keystone seagrass species endemic to the Mediterranean Sea (Hartog and Kuo 2007) where it is considered a biodiversity hotspot. P. oceanica meadows are also the foundation of one of the most characteristic habitats of the Mediterranean Sea (Boudouresque 2004; Boudouresque et al. 2006; Larkum et al. 2006). Its three-dimensional structure creates spawning grounds, nurseries, or permanent habitats for many species, supporting a complex community, which colonises the above- and below-ground plant compartment (including matte) (Bellan-Santini et al. 1986; Borg et al. 2006). Furthermore, P. oceanica meadows act as a carbon sink (blue carbon) mitigating climate change (Pedersen et al. 2011; Pergent-Martini et al. 2021), one of the most valuable ecosystem services for our times (Apostolaki et al. 2011; Marx et al. 2021). However, P. oceanica is sensitive to increasing temperatures, low and high salinities (Boudouresque et al. 2006; Jordà et al. 2012), pollution, and other anthropic pressures (Boudouresque et al. 2006; Jordà et al. 2012; Pazzaglia et al. 2020). Since its ecological status is tightly related to the quality of its surrounding environment, P. oceanica is considered a bioindicator (Montefalcone 2009) and a target of specific conservation and protection measures. At the international level, P. oceanica is protected under the Bern and the Barcelona Conventions, and P. oceanica meadows fall among the habitats of priority interest included in the European Union’s Habitat Directive (92/43/CEE). Moreover, the Marine Strategy Framework Directive (MSFD, 2008/56/EC) selected P. oceanica as a representative species of the angiosperm quality elements for the Mediterranean marine environment.
P. oceanica is a K-strategist, long-lived seagrass species characterised by the very slow growth of its plagiotropic and orthotropic rhizomes (a few centimetres per year; Boudouresque et al. 2006). The species is able to successfully colonise sandy bottoms as well as bare rocky substrates (Den Hartog 1970; Boudouresque and Meinesz 1982; Hemminga and Duarte 2000). Recent studies underlined the influence of the substrate type on the success of P. oceanica seed recruitment and tolerance to hydrodynamic regimes (Alagna et al. 2015; Badalamenti et al. 2015; Montefalcone et al. 2016; Ruju et al. 2018; Zenone et al. 2022).
Seagrasses host a variety of epiphytic organisms, from eukaryotic micro and macroalgae, invertebrates, fungi, viruses, to prokaryotics (Ettinger and Eisen 2019; Mejia et al. 2016; Supaphon et al. 2017; Tarquinio et al. 2021), which may strongly influence the plants’ physiology (Brodersen et al. 2018; Conte et al. 2021a; Crump et al. 2018; Tarquinio et al. 2019; Ugarelli et al. 2017). Hence, each shoot may be considered a network of interactions in which the host and all associated organisms living in/on its tissues establish transient or lasting different relationships, resulting in a complex functional unit, the so-called ‘holobiont’ (sensu Zilber-Rosenberg and Rosenberg 2008). The role of the epiphytic bacterial community and its potential effects on the seagrass ecophysiology has been drawing attention in recent years. It may enhance nutrients availability and uptake (e.g. Garcias-Bonet et al. 2016; Tarquinio et al. 2018; Welsh 2000) and increase seagrass growth by producing growth hormone-like compounds (Celdrán et al. 2012; Conte et al. 2021a; Crump et al. 2018; Tarquinio et al. 2019; Ugarelli et al. 2017; Zilber-Rosenberg and Rosenberg 2008). It can contribute to the host’s defence by producing antimicrobial compounds (Egan et al. 2013; Longford et al. 2019) and by degrading phytotoxic compounds, like H2S and ethanol (Brodersen et al. 2018; Crump et al. 2018; Holmer et al. 2001). In turn, seagrasses provide these epiphytic communities chemically different colonizable surfaces and labile or recalcitrant organic matter (Brodersen et al. 2018; Crump et al. 2018; Martin et al. 2018; Tarquinio et al. 2019; Ugarelli et al. 2017).
Due to the high bacterial turnover, the holobiont is potentially a dynamic entity in which the microbial partner’s composition may change over time and environmental conditions, including changes in host ecophysiology (Mejia et al. 2016; Rotini et al. 2017; Rotini et al. 2020; Tarquinio et al. 2019). The rapid changes in the microbial community structure and composition can facilitate the holobiont’s adaptation to the continuous and unpredictable changes in environmental conditions (Carrier and Reitzel 2017; Duarte et al. 2018); on the other hand, the disruption of the host microbial associations may lead to host pathologic conditions (Bang et al. 2018; Egan et al. 2013; Longford et al. 2019; Martin et al. 2020; Pitlik and Koren 2017; Sullivan et al. 2018). As a consequence, host biology and ecology remain intimately connected with their microbial partners (Mejia et al. 2016; Brodersen et al. 2018). Therefore, identifying the structure and composition of the epiphyte communities is fundamental for improving our understanding of seagrass ecology and establishing more efficient ecosystem management strategies.
Studies on P. oceanica epiphytic bacteria have been performed mainly by culture-dependent approaches (García-Martínez et al. 2009; Marco-Noales et al. 2006); these studies suggested a link between the associated bacterial community and the meadow decline (Carrier and Reitzel 2017; García-Martínez et al. 2009) or the enhancement of leaf growth (Garcias-Bonet et al. 2016). Studies by metagenomic approaches are relatively few and have mainly focused on the roots (Garcias-Bonet et al. 2016; Lehnen et al. 2016; Kohn et al. 2020; Conte et al. 2021a, 2021b). They reported a high N2 fixation and sulphate reduction rate associated with P. oceanica roots. Only a few recent studies focused on the leaf epiphytic bacteria; they underline the potential mutual microbes-seagrass relationship and the variation of seagrass associated with the host condition. Kohn et al. (2020) found an increase in the diversity of the leaf-associated bacterial community with increasing leaf age. In the Cyprus Limassol Bay, in a residual patch of P. oceanica in the proximity of Limassol port, Conte et al. (2021b) found a functional link between plant descriptors and the hosted microbial community. In that study, P. oceanica showed a very high total phenol content, indicating a deteriorated environmental condition and a high relative abundance of bacterial families belonging to the Bulkholderiales order. These bacteria are known degraders of complex C-compounds, including phenols (Nešvera et al. 2015), and their presence indicates how leaf physiology might affect the epiphytic bacteria composition.
The present study is aimed at deepening the knowledge about the bacterial communities associated with P. oceanica leaves along with the plants’ ecophysiological descriptors to explore their potential use for the seagrass health status assessment. Specific objectives of the study were (i) to characterise the associated bacterial communities in meadows growing in two sites around Limassol (Cyprus) and (ii) to evaluate if differences in habitat features (depth, substrate type, turbidity) may affect and change the associated bacterial communities. To this aim, the structure and taxonomic composition of the leaf epiphytic bacterial communities were analysed by 16S rDNA gene analysis; to link these microbial communities with the ecophyisological status of their host, we also analysed morphometric (leaf area, meadow density) and biochemical (pigments, total phenols) descriptors.
Materials and methods
Study area and sampling
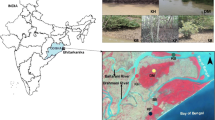
Sampling activities were conducted in December 2017 by SCUBA-diving in two Posidonia oceanica meadows located in the region of the Limassol-Akrotiri Bay (Fig. 1A), which showed different habitat features. Site Ak: Akrotiri-Royal Air Force base (within the Public Access Area (34° 34.83′ N, 33° 2.235′ E) is generally considered a pristine area. Here, the P. oceanica meadow occurs at 7–8 m depth and about 200 m from the shore. It was thick and stood mostly on hard substrate, with some patches growing on soft bottoms. Site Am: Ancient Port of Amathus (34° 42.36′ N, 33° 08.38′ E), a protected archaeological submerged site. The patchy P. oceanica meadow occurs at 1.5–2 m depth and about 50 m from the shore; it stands on a hard substrate (ancient ruins) and is the only meadow left at shallow depth (< 5 m) within the Limassol Bay.
A The two sampling sites in the Limassol-Akrotiri Bay (Cyprus Island, eastern Mediterranean Sea): Akrotiri, Ak, and Amathus, Am (yellow dots; map source: Google Earth, 2020); B schematic representation of Posidonia oceanica shoot composed by rhizome with its roots and leaves (from A. Rotini PhD Thesis, 2011); leaf numbering is indicated from the youngest to the oldest
Seagrass samples were collected on a soft bottom in Ak and on a rocky substrate in Am; as the study was aimed to evaluate if, in ‘healthy’ meadows, habitat features such as depth or substrate may affect/change the seagrass associated bacterial communities, as a first approach, one single sampling event was considered the best option in order to limit the effect of other possible sources of variability. Furthermore, in a framework of ecological ethics, only leaves in three replicates were sampled, and not rhizomes which would entail harming the integrity of the meadows. The second-last leaf in order of emergence within the shoot (a.k.a. second leaf) was chosen for all the analyses (Fig. 1B): it is big enough to allow all the analyses and young enough, not to be affected by senescence processes (Kohn et al. 2020; Iqbal et al. 2023).
For plant descriptor analyses, P. oceanica leaves were haphazardly sampled within each site: the second leaf was cut right above the rhizome from 30 different ramets, at a minimum of 2 m distance from each other, avoiding sampling at the meadow edge. Similarly, for bacterial analyses, the second leaf was cut right above the rhizome from 3 different and randomly chosen shoots, at a minimum of 5 m distance from each other, in each site. The second leaf was chosen in order to study an established bacterial biofilm, avoiding the possible impact of leaf senescence. Each leaf was stored separately underwater, in a ziplock bag to keep the bacterial communities as much as possible unaffected and to separate different replicates.
Three replicates of seawater samples were collected right above the plants (1 L). Three replicates of bulk sediment samples were collected by a mini corer (2.5 cm in diameter and 5 cm in depth). All samples were stored in a cooler until they arrived at the laboratory of the Cyprus University of Technology (within about 30 min) and then kept at 4 °C under dark until sample processing (within 12 h from the sampling). A CTD probe measured temperature, salinity, and pH during plant sampling (Table 1).
Plant and meadow descriptors
Shoot density and biometry
The density of P. oceanica meadow was assessed by counting underwater 3 times the shoots inside a quadrate (20 × 20 cm); shoot density was reported as the number of shoots m−2. All the leaves collected (30) were digitally scanned (Cannon Lide 120) and analysed by the ImageJ platform (version 1.47; Schneider et al. 2012) to calculate the leaf surface area (cm2).
Biochemical analyses
Biochemical analyses were performed on 15 leaves per site, as briefly described below.
Photosynthetic pigments (chlorophyll a and chlorophyll b, total carotenoids) were extracted in duplicate from leaf tissues (250 mg fresh weight, each), grounded in liquid N2 using a mortar and pestle, in 2.5 ml of methanol (4 °C, overnight) according to Wellburn (1994), modified by Rotini et al. (2013a). Quantification of pigments in the extracts was performed with a spectrophotometer (JENWAY 7315, Staffordshire, UK) by measuring the absorption at 470, 652, 665, and 750 nm, and concentrations of these pigments (as mg g−1 of fresh weight) were calculated according to Wellburn (1994).
Phenolic compounds were extracted in duplicate from leaf tissue (100 mg fresh weight, each), grounded in liquid N2 using a mortar and pestle, in 4 ml of 0.1 N HCl (4 °C, overnight) and quantified according to Migliore et al. (2007). The quantification of total phenols was performed in spectrophotometry at 724 nm; concentrations were expressed as chlorogenic acid equivalents (mg) per gramme of plant material (fresh weight, FW).
Bacterial community
At each site, bacterial communities were collected separately from three second P. oceanica leaves, sediment, and bottom seawater samples. In the laboratory, under sterile conditions, each leaf was carefully and repeatedly gently scraped on both sides with a sterile blade sprinkling with a pipette with 2 ml of washing solution, to wash away the biofilm (washing solution: 200 mM Tris–HCl pH 8, 10 mM EDTA, and 0.24% Triton X-100; Kadivar and Stapleton 2003); the solution was then centrifuged (20′, 5000g), and the pellet stored in 2 ml of transport solution (transport solution: Tris 10 mM, EDTA 50 mM; Kadivar and Stapleton 2003) to preserve it, as reported in Mejia et al. (2016). Three sediment samples were stirred and 2 g of mixed sediment per replicate were stored, each submerged in transport solution until DNA extraction. Three seawater samples (1 L per replicate) were collected underwater just above the meadows. In the laboratory, each litre was filtered by a vacuum pump equipped with a sterile 0.2 μm Whatman® membrane filter sterile apparatus. The filters were stored, each submerged in transport solution until DNA extraction.
The bacterial metagenomic DNA was extracted by the Power Soil® DNA isolation kit (Mo Bio, Carlsbad, CA, USA), according to the manufacturer’s instructions. The 16S rRNA gene was amplified by PCR with the universal primers Com1 (forward, 5′-AGCAGCCGCGGTAATAC-3′) and Com2 (reverse, 5′-CGTCAATTCCTTTGAGTTT-3′) that amplify the hypervariable region V3-V4) as reported in Mejia et al. (2016) and Schmalenberger et al. (2001); the amplified DNA was then purified by Gel/PCR Fragment Extraction Kit (GeneAid, Taiwan). The pure DNA extracts were sent to Molecular Research LP (MR DNA Shallowater, Texas, USA) for sequencing by an NGS Illumina MiSeq platform. The raw paired-end sequences were analysed by the Quantitative Insights Into Microbial Ecology (QIIME 2.10; Bolyen et al. 2019) pipeline. The sequences were demultiplexed, quality and chimaera checked, and filtered by the DADA2 QIIME2 plugin (Callahan et al. 2016). As a total, 2258 ASVs (Amplicon Sequence Variants, i.e. each inferred single DNA sequence recovered from a high-throughput analysis of 16S rDNA genes) were identified, with a frequency of 512,299 reads. Taxonomic identification of the 16S rRNA gene sequences was performed using a Naive Bayes classifier trained with the SILVA 138 SSU database (Quast et al. 2012). ASVs classified as chloroplasts or mitochondria were discarded from the dataset. The rarefaction curves, built to evaluate differences and efficiency in the sampling effort, confirmed that the sequencing coverage was good (see Supplementary Fig. S1). The dataset was normalised at the common depth of 15,567 sequences per sample, the lowest number of sequences in the dataset (sample P. oceanica leaves, site A, replicate #1). Although this imposed a low sequence number, it allowed keeping three biological replicates of each sample type in the dataset. The final dataset (cleaned from chloroplasts and mitochondria sequences and normalised) was composed of a total of 2187 ASVs, used to perform the downstream analyses (Tab. S1). Statistical analyses were performed within QIIME (Bolyen et al. 2019) or PAST 4.05 (Hammer et al. 2001).
This Targeted Locus Study project has been deposited at GenBank as Bioproject PRJNA916897.
Statistical analyses
Differences in leaf area (n = 31), meadow density (n = 3) or pigments-total phenols contents (n = 12) were evaluated by Student’s t-test.
Bacterial diversity within samples (α-diversity) was estimated using Shannon-Wiener Index (PAST 4.05; Hammer et al. 2001; Legendre and Legendre 1998). Pearson correlation (QIIME2, alpha correlation plugin; Bolyen et al. 2019; Pearson 1895) was used to test the possible relationship between seagrass leaf bacterial α-diversity and leaf biochemical parameters. Stratified permutational multivariate analysis of variance (Adonis R Vegan function; Oksanen et al. 2020) with Bray-Curtis dissimilarity was used to evaluate significant differences of β-diversity in the whole dataset using sites and matrices as a source of variance. These data were visualised by PCoA (QIIME2; Bolyen et al. 2019; Halko et al. 2011). To detect finer differences, each sample type was compared by one-way ANOSIM with Bray-Curtis dissimilarity (PAST 4.05; Legendre and Legendre 2012; Hammer et al. 2001).
Venn diagrams were built to visualise shared and unique ASVs in leaf-associated bacterial communities and to identify the bacterial core (the shared component; https://bioinformatics.psb.ugent.be/webtools/Venn/). Bar plots were used to visualise the bacterial core agglomerated at the family level. The analysis of the composition of microbiomes (ANCOM; Mandal et al. 2015) was used on the dataset agglomerated at the family level to detect significant differences in the distribution of the bacterial communities associated with seagrass leaves. The thorough list of the leaf-associated bacteria in each replicate, agglomerated at the family level, was used to build the heatmap (PAST 4.05, Hammer et al. 2001, visualised in Excel).
Results and discussion
The ecological status of Posidonia oceanica was evaluated in meadows from two sites of the Cyprus Island, where environmental conditions are different (§ 2.1; Fig. 1A). To this end, morpho-physiological seagrass descriptors together with the composition of the associated bacterial communities were analysed in each site.
Morpho-physiological descriptors are widely used to identify the health status of seagrass plants (i.e. Winters et al. 2011; Rotini et al. 2013a; Schubert et al. 2015; Collier et al. 2009; Ceccherelli et al. 2018; Beca-Carretero et al. 2019). In this study, a comparable shoot density was observed in the two sites (Table 2), although slightly higher in Akrotiri than in Amathus. Differences were not significant (Student’s t-test, P > 0.05) and the average density values (< 400 shoots/m2) account for dense meadows in both sites (classification of Giraud 1997, modified by Pergent et al. 1995). Also, leaf total phenol content did not differ between the two sites and showed quite low values. Again, this indicates a healthy and comparable ecological status of the two meadows, as the phenol content is a seagrass descriptor is known to increase under stressed conditions (Dumay et al. 2004; Migliore et al. 2007; Rotini et al. 2013a, 2013b; Ceccherelli et al. 2018; Mannino and Micheli 2020; Conte et al. 2023). Plants from both Amathus and Akrotiri sites were found to contain half the total phenol content of their counterparts growing in the polluted area of the Limassol port (Conte et al. 2021b). Furthermore, the concentrations found in this study are comparable to those already recorded in meadows from pristine sites (Fresi et al. 2004; Costa et al. 2015). Some differences were found in leaf biometry and photosynthetic pigment content, both showing higher values in Amathus (Am) than in Akrotiri (Ak), even though Am is the shallowest site. These differences probably depend on the different local light regimes (high turbidity and strong currents in Amathus; see Table 1), but both these light regimes did not represent a stressing condition for plants, being comparable in the two sites the value of chlorophyll a/b ratio, a marker of light stress (Casazza and Mazzella 2002). Overall, the morpho-physiological seagrass descriptors depict a similar and balanced plant ecophyisological status of both P. oceanica meadows, despite the geomorphological differences between sites (e.g. depth, substrate, and turbidity), but in agreement with the good ecological conditions of both sites (e.g. lack of pollutants or anthropic pressure).
Seagrass and associated bacterial communities are considered a dynamic unity, the seagrass holobiont, and the structure and composition of the bacterial communities change with the environmental conditions and/or plant traits, helping plants to cope with environmental changes (Conte et al. 2021a). Because of this tight relationship, the alteration of the bacterial communities may be marker and/or responsible for damaged conditions of the host (Bang et al. 2018; Egan et al. 2013; Longford et al. 2019; Martin et al. 2020; Pitlik and Koren 2017; Sullivan et al. 2018). In the two meadows of Cyprus, consistently with results from plant descriptors, a comparable pattern was found in the bacterial communities associated with P. oceanica plants: the α-diversity of the bacterial community showed comparable values in the two sites. Generally, the bacterial diversity of both seawater and P. oceanica leaves was higher in Ak, while sediment bacterial α-diversity was higher in Am (Table 3). As expected, an overall significant difference in the bacterial communities’ structure and composition (β-diversity) was found among sample types (i.e. seawater, sediment, and leaves) and sites (ADONIS, P < 0.05; Fig. 2). No significant differences in β-diversity were found between the bacterial community of the different sample types between sites, except for the sediment (ANOSIM, P < 0.05); this was expected, due to the intrinsic differences in substrate types: a soft bottom in Ak and a rocky substrate in Am (Vasquez M., personal comm.), with different characteristics and available microenvironments in the two colonizable substrates. By comparing leaf-associated and seawater or sediment bacteria, no differences were found between leaf-associated and seawater bacterial community in both sites (β-diversity; ANOSIM, P > 0.05), and this was expected because the seawater free-living bacteria and those associated with suspended particles represent the bacterial microbial pool, and the suspended particles are considered the main source of leaf colonisers (Fahimipour et al. 2017; Iqbal et al. 2021, 2023).
The taxonomic composition of the bacterial communities found in different sample types was evaluated as Amplicon Sequence Variants (ASVs), using the normalised dataset agglomerated at the family level. The leaf-associated bacterial communities evaluated at family level displayed similar structure and composition (Fig. 3). Leaf-associated bacterial communities showed a common dominant component in the two sites (Fig. 4), i.e. a high number of shared ASVs: 100 shared ASVs were found in the communities from the two sites, accounting for 81% (37,827 reads) and 78% (36,659 reads) ASVs from Ak and Am, respectively. These shared ASVs represent the bacterial core of both communities. The unique ASVs found in each leaf community accounted for 19% (8874 reads) and 21% (10,042 reads) in Am and Ak, respectively, representing the environmental ‘fingerprint’, i.e. the peculiar bacteria linked to the specific conditions of each site, the site-specific bacterial colonisers (Fig. 4A). In both sites, the P. oceanica bacterial core (i.e. the core micobiome) was composed of Thalassospiraceae, Microtrichaceae, Enterobacteriaceae, Saprospiraceae, and Hyphomonadaceae families (Fig. 4B). Among them, some are known as marine biofilm-forming bacteria such as Thalassospiraceae, an aerobic chemoorganotrophic bacterial family with the ability to reduce nitrate (Imhoff and Wiese 2014); others, like Microtrichaceae and Hyphomonadaceae, are potentially involved in leaf nitrate supply (Abraham and Rohde 2014; Korlević et al. 2021; Szitenberg et al. 2022). Alongside, abundant families were Saprospiraceae, known to break down complex organic carbon (McIlroy and Nielsen 2014), and Enterobacteriaceae, widespread ammonifying bacteria (Rehr and Klemme 1989). Thus, the dominant component of the bacterial core is likely involved in pivotal basic processes for the host plant as, among others, nitrogen cycling. Slight differences were found in the unique components, with very few bacterial families uniquely associated with the plants of each site: in Ak 7, unique bacterial families were found at low percentages (< 3%; Cellvibrionaceae, Alteromonadaceae, Moraxellaceae, Pseudomonadaceae, Xanthomonadaceae, Shewanellaceae, and Spongiibacteraceae), and in Am 4, unique bacterial families were found at low percentages (< 3%), three classified at order and one at class level (ASV that have not been recognised further in the taxonomic rank). These bacteria belong to Pirellulaceae, Propionibacteriaceae, Flavobacteriaceae, Hyphomicrobiaceae, Entomoplasmatales, Chitinophagales, Alphaproteobacteria, and two other unknown families. The composition comparison of leaf-associated microbiomes (ANCOM) did not highlight significant differences in the bacterial families’ distribution. These results, in the holobiont perspective, confirm that under comparable good environmental conditions—in spite of geomorphological differences—leaf-associated bacterial communities are similar and involved in plants in basic processes; hence, they suggest to be related to the healthy status of seagrass plants in both sites.
Results from the present study combined with those from a recent study performed in the same area (Limassol-Akrotiri Bay), on the same dates and with the same technical protocols (Conte et al. 2021b), further support the functional link between seagrass plant traits and their associated bacterial communities. In this study, the comparable structure and composition of leaf-associated P. oceanica bacterial community superimposes with the similar eco-physiological status of plants in the two meadows, notwithstanding the different habitat features of the two sites. Conversely, in the area of the Limassol port, investigated by Conte et al. (2021b), which is considered polluted and located in between the two undisturbed meadows from this study (as indicated by the red dot in Fig. 1A), P. oceanica plants displayed ecophysiological signs of stress, i.e. a very high total phenol content in leaves (twice the amount of Amathus and Akrotiri plants) and associated bacterial communities that were completely different from the associated bacterial communities on undisturbed meadows. In the Limassol port, the plants’ stressed condition was mirrored by an ad hoc composition of the epiphytic bacterial community: P. oceanica leaves hosted bacteria of Bulkholderiales order, at relatively high abundance (Conte et al. 2021b); this order includes families able to degrade phenols (Nešvera et al. 2015). Again, the high concentrations of total phenols and the presence of Bulkholderiales in the Limassol port plants supported the tight relationship between seagrasses and their leaf-associated bacterial communities.
Conclusion
In conclusion, this study provides new insights into the knowledge of the bacterial communities associated with the iconic seagrass P. oceanica, which until now has been very little explored.
The ecophysiological seagrass descriptors applied here alongside our molecular work, depicted a similar and good plant conservation status in the two sites, despite the differences in habitat features (substrate type, depth, turbidity). The similar seagrass ecophysiology between the two different sites, resulted in a similar recruitment of bacterial communities, confirming that ecophysiological conditions, rather than habitat features, shape the seagrass associated epiphytic microbial community. As already observed by Conte et al. (2021b), seagrass showed ‘elective affinities’ with their associated bacteria, further supporting the tight and functional relationship plant/bacteria and the bacterial involvement in plant homeostasis.
Furthermore, the two sites, in spite of geomorphological differences, can be considered pristine sites with comparable good environmental conditions: in the holobiont perspective, the occurrence of the same bacterial core strengthens the assumption of their functional role, supporting the use of associated bacteria as an important source of ecological information and a putative seagrass health descriptor.
Data availability
The sequences have been deposited in GenBank as Targeted Locus Sequences under the BioProject ID PRJNA916897.
References
Abraham WR, Rohde M (2014) The family Hyphomonadaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30197-1_260
Alagna A, Fernández TV, Anna GD, Magliola C, Mazzola S, Badalamenti F (2015) Assessing Posidonia oceanica seedling substrate preference: an experimental determination of seedling anchorage success in rocky vs sandy substrates. PLoS One 10(4):e0125321
Apostolaki ET, Holmer M, Marbà N, Karakassis I (2011) Reduced carbon sequestration in a Mediterranean seagrass (Posidonia oceanica) ecosystem impacted by fish farming. Aq Env Interac 2(1):49–59
Badalamenti F, Alagna A, Fici S (2015) Evidences of adaptive traits to rocky substrates undermine paradigm of habitat preference of the Mediterranean seagrass Posidonia oceanica. Sci Rep 5(1):1–6
Bang C, Dagan T, Deines P, Dubilier N, Duschl WJ, Fraune S, Bosch TC (2018) Metaorganisms in extreme environments: do microbes play a role in organismal adaptation? Zoology 127:1–19
Beca-Carretero P, Guihéneuf F, Winters G, Stengel DB (2019) Depth-induced adjustment of fatty acid and pigment composition suggests high biochemical plasticity in the tropical seagrass Halophila stipulacea. Mar Ecol Prog Ser 608:105–177. https://doi.org/10.3354/meps12816
Bellan-Santini D, Willsie A, Arnoux A (1986) Distribution comparée des crustaces amphipods de la matte d’herbier de posidonies mort et vivant. Rapp Comm Int Mer Med 30(8)
Bolyen E, Rideout JR, Dillon MR, Bokulich NA et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Borg JA, Rowden AA, Attrill MJ, Schembri PJ, Jones MB (2006) Wanted dead or alive: high diversity of macroinvertebrates associated with living and ‘dead’ Posidonia oceanica matte. Mar Biol 149(3):667–677
Boudouresque CF (2004) Marine biodiversity in the Mediterranean: status of species, populations and communities. Travaux Sci Parc Natl Port-Cros 20:97–146
Boudouresque CF, Meinesz A (1982) Découverte de l’herbier de Posidonies. Cahier Parc Nat Port-Cros 4:1–79
Boudouresque CF, Mayot N, Pergent G (2006) The outstanding traits of the functioning of the Posidonia oceanica seagrass ecosystem. Biol Mar Medit 13(4):109–113
Brodersen KE, Siboni N, Nielsen DA, Pernice M, Ralph PJ, Seymour J, Kühl M (2018) Seagrass rhizosphere microenvironment alters plant-associated microbial community composition. Environ Micro 20(8):2854–2864
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Method 13(7):581–583
Carrier TJ, Reitzel AM (2017) The hologenome across environments and the implications of a host-associated microbial repertoire. Front Microbiol 8:802
Casazza G, Mazzella L (2002) Photosynthetic pigment composition of marine angiosperms: preliminary characterization of Mediterranean seagrasses. Bull Mar Sci 71(3):1171–1181
Ceccherelli G, Oliva S, Pinna S, Piazzi L, Procaccini G, Marin-Guirao L, Bulleri F (2018) Seagrass collapse due to synergistic stressors is not anticipated by phenological changes. Oecol 186(4):1137–1152
Celdrán D, Espinosa E, Sánchez-Amat A, Marín A (2012) Effects of epibiotic bacteria on leaf growth and epiphytes of the seagrass Posidonia oceanica. Mar Ecol Prog Ser 456:21–27
Collier CJ, Lavery PS, Ralph PJ, Masini RJ (2009) Shade-induced response and recovery of the seagrass Posidonia sinuosa. J Exp Mar Biol Ecol 370:89–103. https://doi.org/10.1016/j.jembe.2008.12.003
Conte C, Rotini A, Manfra L, D’Andrea MM, Winters G, Migliore L (2021a) The seagrass holobiont: what we know and what we still need to disclose for its possible use as an ecological indicator. Water 13(4):406
Conte C, Rotini A, Winters G, Vasquez MI, Piazza G, Kletou D, Migliore L (2021b) Elective affinities or random choice within the seagrass holobiont? The case of the native Posidonia oceanica (L) Delile and the exotic Halophila stipulacea (Forssk) Asch from the same site (Limassol, Cyprus). Aq Bot 174:103420
Conte C, Apostolaki ET, Vizzini S, Migliore L (2023) A tight interaction between the native seagrass Cymodocea nodosa and the exotic Halophila stipulacea in the aegean sea highlights seagrass holobiont variations. Plants 12(2):350. https://doi.org/10.3390/plants12020350
Costa MM, Barrote I, Silva J, Olivé I, Alexandre A, Albano S, Santos R (2015) Epiphytes modulate Posidonia oceanica photosynthetic production, energetic balance, antioxidant mechanisms, and oxidative damage. Front Mar Sci 2:111. https://doi.org/10.3389/fmars.2015.00111
Crump BC, Wojahn JM, Tomas F, Mueller RS (2018) Metatranscriptomics and amplicon sequencing reveal mutualisms in seagrass microbiomes. Front Microbiol 9:388
Den Hartog C (1970) The seagrasses of the world. North Holland Publishing Co., Amsterdams
Duarte B, Martins I, Rosa R, Matos AR, Roleda MY, Reusch TB, Jueterbock A (2018) Climate change impacts on seagrass meadows and macroalgal forests: an integrative perspective on acclimation and adaptation potential. Front Mar Sci 5:190
Dumay O, Costa J, Desjobert JM, Pergent G (2004) Variations in the concentration of phenolic compounds in the seagrass Posidonia oceanica under conditions of competition. Phytochem 65(24):3211–3220
Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T (2013) The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiol Rev 37(3):462–476
Ettinger CL, Eisen JA (2019) Characterization of the mycobiome of the seagrass, Zostera marina, reveals putative associations with marine chytrids. Front Microbiol 10:2476
Fahimipour AK, Kardish MR, Lang JM, Green JL, Eisen JA, Stachowicz JJ (2017) Global-scale structure of the eelgrass microbiome. App Env Microbiol 83(12):e03391–e03316
Fresi E, Dolce T, Forni C, Lorenzi C, Migliore L, Rizzelli D Scardi M (2004) La prateria di Posido-nia oceanica (L) Delile di Talamone (Grosseto, Italia): struttura e stato di salute, Conference: Le scienze naturali, economiche e giuridiche nello studio e per la gestione degli ambienti acquatici, 18-22 October 2004; Terrasini, Palermo, CONISMA-AIOL
García-Martínez M, López-López A, Calleja ML, Marbà N, Duarte CM (2009) Bacterial community dynamics in a seagrass (Posidonia oceanica) meadow sediment. Estuar Coast 32(2):276–286
Garcias-Bonet N, Arrieta JM, Duarte CM, Marbà N (2016) Nitrogen-fixing bacteria in Mediterranean seagrass (Posidonia oceanica) roots. Aq Bot 131:57–60
Giraud G (1997) Essai de classement des herbiers de Posidonia oceanica (Linné) Delile. Bot Mar 20(8):487–492. https://doi.org/10.1515/botm.1977.20.8.487
Halko N, Martinsson P-G, Shkolnisky Y, Tygert M (2011) An algorithm for the principal component analysis of large data sets. SIAM J Sci Comp 33(5):2580–2594. https://doi.org/10.1137/100804139
Hammer Ø, Harper DA, Ryan P (2001) D PAST: paleontological statistics software package for education and data analysis. Palaeont Electro 4(1):9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Hartog C, Kuo J (2007) Taxonomy and biogeography of seagrasses. In: Larkum AW, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 1–23
Hemminga MA, Duarte CM (2000) Seagrass ecology. Cambridge University Press, Cambridge (UK)
Holmer M, Andersen FØ, Nielsen SL, Boschker HT (2001) The importance of mineralization based on sulfate reduction for nutrient regeneration in tropical seagrass sediments. Aq Bot 71(1):1–17
Imhoff JF, Wiese J (2014) The order Kiloniellales. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt S, Thompson F (eds) The prokaryotes. Springer, Berlin, pp 301–306
Iqbal MM, Nishimura M, Haider MN, Sano M, Ijichi M, Kogure K (2021) Diversity and composition of microbial communities of an eelgrass (Zostera marina) bed in Tokyo Bay, Japan. Microbes Environ 36:ME21037. https://doi.org/10.1264/jsme2.ME21037
Iqbal MM, Nishimura M, Haider MN, Yoshizawa S (2023) Microbial communities on eelgrass (Zostera marina) thriving in Tokyo Bay and the possible source of leaf-attached microbes. Front. Microbiol 13:1102013. https://doi.org/10.3389/fmicb.2022.1102013
Jordà G, Marbà N, Duarte CM (2012) Mediterranean seagrass vulnerable to regional climate warming. Nat Clim Change 2(11):821–824
Kadivar H, Stapleton AE (2003) Ultraviolet radiation alters maize phyllosphere bacterial diversity. Microb Ecol 45(4):353–361 http://www.jstor.org/stable/4287713
Kohn T, Rast P, Kallscheuer N, Wiegand S, Boedeker C, Jetten MS, Jogler C (2020) The microbiome of Posidonia oceanica seagrass leaves can be dominated by Planctomycetes. Front Microbiol 11:1458. https://doi.org/10.3389/fmicb.2020.01458
Korlević M, Markovski M, Zhao Z, Herndl GJ, Najdek M (2021) Seasonal dynamics of epiphytic microbial communities on marine macrophyte surfaces. Front Microbiol 12:671342. https://doi.org/10.3389/fmicb.2021.671342
Larkum AW, Orth RJ, Duarte CM (2006) Seagrasses: biology, ecology and conservation. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-2983-7
Legendre P, Legendre L (1998) Numerical ecology, 2nd English edn. Elsevier, Amsterdam
Legendre P, Legendre L (2012) Numerical ecology, Third edn. Elsevier, Amsterdam, p 1998
Lehnen N, Marchant HK, Schwedt A, Milucka J, Lott C, Weber M, Kuypers MM (2016) High rates of microbial dinitrogen fixation and sulfate reduction associated with the Mediterranean seagrass Posidonia oceanica. Syst App Microbiol 39(7):476–483
Longford SR, Campbell AH, Nielsen S, Case RJ, Kjelleberg S, Steinberg PD (2019) Interactions within the microbiome alter microbial interactions with host chemical defences and affect disease in a marine holobiont. Sci Rep 9(1):1–13
Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD (2015) Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis 26(1):27663
Mannino AM, Micheli C (2020) Ecological function of phenolic compounds from mediterranean fucoid algae and seagrasses: an overview on the genus Cystoseira sensu lato and Posidonia oceanica (L) Delile. J Mar Sci Engin 8(1):19
Marco-Noales E, Ordax M, Delgado A, López MM, Saavedra MJ, Martínez-Murcia A, Duarte CM (2006) Microbiota associated with Posidonia oceanica in Western Mediterranean Sea. Mod Multidiscipl Appl Microbiol:114–119. https://doi.org/10.1002/9783527611904.ch20
Martin BC, Alarcon MS, Gleeson D, Middleton JA, Fraser MW, Ryan MH, Kilminster K (2020) K Root microbiomes as indicators of seagrass health. FEMS Microbiol Ecol 96(2):fiz201
Martin BC, Gleeson D, Statton J, Siebers AR, Grierson P, Ryan MH, Kendrick GA (2018) Low light availability alters root exudation and reduces putative beneficial microorganisms in seagrass roots. Front Microbiol 8:2667
Marx L, Flecha S, Wesselmann M, Morell C, Hendriks IE (2021) Marine macrophytes as carbon sinks: comparison between seagrasses and the non-native alga Halimeda incrassata in the Western Mediterranean (Mallorca). Front Mar Sci 8:746379. https://doi.org/10.3389/fmars.2021.746379
McIlroy SJ, Nielsen PH (2014) The family Saprospiraceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt S, Thompson F (eds) The prokaryotes. Springer, Berlin, pp 863–889
Mejia AY, Rotini A, Lacasella F, Bookman R, Thaller MC, Shem-Tov R, Migliore L (2016) Assessing the ecological status of seagrasses using morphology, biochemical descriptors and microbial community analyses. A study in Halophila stipulacea (Forsk) Aschers meadows in the northern Red Sea. Ecol Ind 60:1150–1163
Migliore L, Rotini A, Randazzo D, Albanese NN, Giallongo A (2007) Phenols content and 2-D electrophoresis protein pattern: a promising tool to monitor Posidonia meadows health state. BMC Ecol 7(1):1–8
Montefalcone M (2009) Ecosystem health assessment using the Mediterranean seagrass Posidonia oceanica: a review. Ecol Indic 9(4):595–604
Montefalcone M, Vacchi M, Carbone C, Cabella R, Schiaffino CF, Elter FM, Ferrari M (2016) Seagrass on the rocks: Posidonia oceanica settled on shallow-water hard substrata withstands wave stress beyond predictions. Est Coast Shelf Sci 180:114–122
Nešvera J, Rucká L, Pátek M (2015) Catabolism of phenol and its derivatives in bacteria: genes, their regulation, and use in the biodegradation of toxic pollutants. Ad App Microbiol 93:107–160
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, et al. (2020) vegan: community ecology package. R package version 2.5-7. https://cran.r-project.org/package=vegan. Accessed Nov 2022
Pazzaglia J, Santillán-Sarmiento A, Helber SB, Ruocco M, Terlizzi A, Marín-Guirao L, Procaccini G (2020) Does warming enhance the effects of eutrophication in the seagrass Posidonia oceanica? Front Mar Sci 7:564805. https://doi.org/10.3389/fmars.2020.564805
Pearson K (1895) Note on regression and inheritance in the case of two parents. Proceed Royal Soc Lon 58:240–242
Pedersen MØ, Serrano O, Mateo MÁ, Holmer M (2011) Temperature effects on decomposition of a Posidonia oceanica mat. Aq Microb Ecol 65(2):169–182
Pergent G, Pergent-Martini C, Boudouresque CF (1995) Utilisation de l'herbier à Posidonia oceanica comme indicateur biologique de la qualité du milieu littoral en Méditerranée: état des connaissances. Mésogée (Marseille) 54:3–27
Pergent-Martini C, Pergent G, Monnier B, Boudouresque CF, Mori C, Valette-Sansevin A (2021) A contribution of Posidonia oceanica meadows in the context of climate change mitigation in the Mediterranean Sea. Mar Env Res 165:105236
Pitlik SD, Koren O (2017) How holobionts get sick-toward a unifying scheme of disease. Microbiome 5(1):1–4
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41(D1):D590–D596
Rehr B, Klemme JH (1989) Competition for nitrate between denitrifying Pseudomonas stutzeri and nitrate ammonifying enterobacteria. FEMS Microbiol Ecol 5(1):51–57
Rotini A, Belmonte A, Barrote I, Micheli C, Peirano A, Santos RO, Migliore L (2013a) Effectiveness and consistency of a suite of descriptors for assessing the ecological status of seagrass meadows (Posidonia oceanica L Delile). Est Coast Shelf Sci 130:252–259
Rotini A, Anello L, Di Bernardo M, Giallongo A, Valiante L, Migliore L (2013b) Comparative analysis of bed density, total phenol content and protein expression pattern in Posidonia oceanica (L) Delile. Open J Ecol 3(6):438–444
Rotini A, Mejia AY, Costa R, Migliore L, Winters G (2017) Ecophysiological plasticity and bacteriome shift in the seagrass Halophila stipulacea along a depth gradient in the Northern Red Sea. Front Plant Sci 7:2015
Rotini A, Conte C, Seveso D, Montano S, Galli P, Vai M, Mejia A (2020) Daily variation of the associated microbial community and the Hsp60 expression in the Maldivian seagrass Thalassia hemprichii. J Sea Res 156:101835
Ruju A, Ibba A, Porta M, Buosi C, Passarella M, De Muro S (2018) The role of hydrodynamic forcing, sediment transport processes and bottom substratum in the shoreward development of Posidonia oceanica meadow. Estuar Coast Shelf Sci 212:63–72
Schmalenberger A, Schwieger F, Tebbe CC (2001) Effect of primers hybridizing to different evolutionarily conserved regions of the small-subunit rRNA gene in PCR-based microbial community analyses and genetic profiling. Appl Environ Microbiol 67:3557–3563. https://doi.org/10.1128/AEM.67.8.3557
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Schubert N, Colombo-Pallota MF, Enríquez S (2015) Leaf and canopy scale characterization of the photoprotective response to high-light stress of the seagrass Thalassia testudinum. Limnol Oceanogr 60:286–302. https://doi.org/10.1002/lno.10024
Sullivan BK, Trevathan-Tackett SM, Neuhauser S, Govers LL (2018) Host-pathogen dynamics of seagrass diseases under future global change. Mar Poll Bull 134:75–88
Supaphon P, Phongpaichit S, Sakayaroj J, Rukachaisirikul V, Kobmoo N, Spatafora JW (2017) Phylogenetic community structure of fungal endophytes in seagrass species. Bot Mar 60(4):489–501
Szitenberg A, Beca-Carretero P, Azcárate-García T, Yergaliyev T, Alexander-Shani R, Winters G (2022) Teasing apart the host-related, nutrient-related and temperature-related effects shaping the phenology and microbiome of the tropical seagrass Halophila stipulacea. Env Microbio 17(1):1–17. https://doi.org/10.1186/s40793-022-00412-6
Tarquinio F, Bourgoure J, Koenders A, Laverock B, Säwström C, Hyndes GA (2018) Microorganisms facilitate uptake of dissolved organic nitrogen by seagrass leaves. ISME J 12(11):2796–2800. https://doi.org/10.1038/s41396-018-0218-6
Tarquinio F, Hyndes GA, Laverock B, Koenders A, Säwström C (2019) The seagrass holobiont: understanding seagrass-bacteria interactions and their role in seagrass ecosystem functioning. FEMS Microbiol Let 366(6):fnz057. https://doi.org/10.1093/femsle/fnz057
Tarquinio F, Attlan O, Vanderklift MA, Berry O, Bissett A (2021) Distinct endophytic bacterial communities inhabiting seagrass seeds. Front Microb 12:703014
Ugarelli K, Chakrabarti S, Laas P, Stingl U (2017) The seagrass holobiont and its microbiome. Microorg 5(4):81. https://doi.org/10.3390/microorganisms5040081
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physio 144(3):307–313
Welsh DT (2000) Nitrogen fixation in seagrass meadows: regulation, plant-bacteria interactions and significance to primary productivity. Ecol Lett 3:58–71
Winters G, Nelle P, Fricke B, Rauch G, Reusch TBH (2011) Effects of a simulated heat wave on photophysiology and gene expression of high- and low-latitude populations of Zostera marina. Mar Ecol Prog Ser 435:83–95. https://doi.org/10.3354/meps09213
Zenone A, Badalamenti F, Alagna A, Gorb SN, Infantes E (2022) Assessing tolerance to the hydrodynamic exposure of Posidonia oceanica seedlings anchored to rocky substrates. Front Mar Sci. https://doi.org/10.3389/fmars.2021.788448
Zilber-Rosenberg I, Rosenberg E (2008) Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev 32(5):723–735
Acknowledgements
The authors would like to thank the MER Laboratory members Periklis Kleitou, Charalampos Antoniou, and Ioannis Savva for their help in the fieldwork and their knowledge of the site; Oswaldo E. Vasquez Ravelo, ATEMAR, DR, for its support during the fieldwork; and Stylianos Kanakaris for support during the lab experiments at the Cyprus University of Technology.
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement. Alice Rotini benefited from a Short Term Scientific Mission grant from the COST Action CA 15121 ‘Advancing Marine Conservation in the European and Contiguous Seas – Mar Cons’ to travel to Cyprus and conduct sampling activities and laboratory experiments (COST-STSM- CA15121-39495); Chiara Conte was funded by a 3-year PhD grant from the Tor Vergata Rome University (XXXIV cycle), Doctorate in Evolutionary Biology and Ecology. Data elaboration has been performed, thanks to the ISCRA CINECA Project Class C on Galileo100 server.
Author information
Authors and Affiliations
Contributions
Investigation: AR, CC, GW, MIV, and LM; resources: MIV and LM; data curation: AR and CC; bioinformatics analysis: CC; writing—original draft preparation: AR and CC; writing—review and editing: AR, CC, GW, MIV, and LM; supervision: LM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alice Rotini and Chiara Conte contributed equally to this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rotini, A., Conte, C., Winters, G. et al. Undisturbed Posidonia oceanica meadows maintain the epiphytic bacterial community in different environments. Environ Sci Pollut Res 30, 95464–95474 (2023). https://doi.org/10.1007/s11356-023-28968-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28968-x