Abstract
A new strategy for ultramicroporous activated carbons production from avocado seeds was developed. Combined solvothermal carbonization and thermal KOH activation were conducted. Solvothermal carbonizations were performed in a stainless-steel autoclave lined with Teflon at the temperature of 180 °C for 12 h in three different liquids (water, methanol, isopropyl alcohol). Chars were activated by KOH. The carbonization combined with activation took place in the oven at 850 °C for 1 h. All the samples were very good CO2 sorbents. The highest CO2 adsorption at a pressure of 1 bar was achieved for activated carbon produced using isopropanol. The best carbon dioxide adsorption was equal to 6.47 mmol/g at 0 °C and 4.35 mmol/g at 20 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emissions of anthropogenic CO2 have caused a significant impact on the global climate (Zhang et al. 2019). CO2 is mainly produced by fossil fuel combustion but also accompanies cement production, petrochemical, and other chemical processes (Huang et al. 2022). Activated carbons produced from waste biomass are auspicious and low-cost materials for CO2 adsorption (Creamer et al. 2014).
Some methods of activated carbons production from avocado seeds have been described in the literature. Avocado seeds were applied for activated carbon production for phenol removal from water (Rodrigues et al. 2011). The carbonization was performed in an oven at 800oC and then activated with CO2 at 900 °C. Carbon material with a low specific surface area (206 m2/g) and a negligible volume of mesopores (0.048 cm3/g) and micropores (0.052 cm3/g) were obtained.
The procedure of activated carbon synthesis in a microwave oven using ZnCl2 as an activator was described (Leite et al. 2017). A material with a relatively high specific surface area (1432 m2/g) and a low volume of mesopores (0.325 cm3/g) and micropores (0.119 cm3/g) were obtained. Such properties allowed this material to be used as a sorbent of resorcinol and 3-aminophenol from aqueous solutions.
The procedure of activated carbons production from avocado seeds by pyrolysis at the temperature range 500–700 °C and activation by ZnCl2 was presented (Leite et al. 2018). Materials with a relatively high specific surface area (1122–1584 m2/g), medium mesopore volume (0.475–0.691 cm3/g), and low micropore volume (0.084–0.156 cm3/g) were obtained. Such properties allowed the use of these activated carbons as sorbents for amoxicillin, caffeine, captopril, enalapril, and meloxicam.
As an activating agent in the synthesis of activated carbons from avocado seeds, sulfuric acid was used at a temperature of 100 °C (Bhaumik et al. 2014). A material with a low specific surface area (14 m2/g) and a low pore volume 0.0323 cm3/g was received. This material was used for the adsorption of Cr(VI) ions from aqueous solutions.
The method of activated carbons production based on activation with H3PO4 at the temperature range 800–1000 °C was described (Elizalde-González et al. 2007). The material with the highest blue 41 dye adsorption had a low surface area (143 m2/g) and a low pore volume 0.073 cm3/g.
The production of activated carbons from avocado seeds by carbonization in nitrogen or carbon dioxide at 600–1000 °C was presented (Salomón-Negrete et al. 2018). The obtained materials exhibited low specific surface area (52–300 m2/g), low pore volume (0.051–0.172 cm3/g), and especially micropores (0.019–0.122 cm3/g). Such properties allowed using these activated carbons as sorbents of fluorine ions from aqueous solutions.
To the best of our knowledge, avocado seed as a source of activated carbon has been described, as of today, only in six publications listed above. The above review of the literature clearly showed that so far, it has not been possible to develop a method of activated carbons production from avocado seeds with high microporosity, which is essential for sorbents with high CO2 adsorption.
In this work, we reported for the first time avocado seeds as carbon precursors for CO2 sorbents. A new strategy for ultramicroporous activated carbons production from avocado seeds was developed. We demonstrate that by a combination of solvothermal carbonization and thermal KOH activation allowed to produce activated carbons with uniform ultramicropores (∼ 0.50 nm) and with the enhancement of CO2 adsorption. Under 1 bar, the CO2 adsorption at a temperature of 0 °C ranged from 6.47 to 6.31 mmol/g and at 20 °C from 4.13 to 4.13 mmol/g. According to our knowledge, these values are very high.
Materials and methods
Materials
The carbon precursor, avocado seeds, were bought from supermarkets in Poland. The following reagents were purchased from Chempur (Piekary Śląskie, Poland): methanol, KOH, HCl 35–38%. Isopropyl alcohol was provided by P.P.H. Stanlab Sp. Z o. o. (Lublin, Poland). All chemicals mentioned above were of analytical grade.
Nitrogen (99.999% purity) and carbon dioxide (99.999% purity), obtained from Messer Polska Sp. z o. o., were used for adsorption and samples characterization.
Activated carbon synthesis
The dried avocado seeds were fine powdered. Solvothermal carbonizations of avocado seed in three different liquids (water, methanol, isopropyl alcohol ) were performed in a stainless-steel autoclave lined with Teflon at the temperature of 180 °C for 12 h. The resulting chars were washed with deionized water and dried at 190 °C.
The char was mixed with saturated KOH solution. The mass ratio of char/pure KOH was 1:1. The mixture was left for 3 h and then dried at 190 °C.
The dried mixtures were placed in a tubular furnace and heated to 850 °C under nitrogen flow. The carbonization combined with activation took place in the oven for 1 h. Then, samples were washed with deionized water until neutral pH was achieved. In the end, samples were dried at 190 °C.
Characterization of the material
To obtain textural properties, nitrogen sorption isotherms at 77 K were investigated. The relative pressure from 9 × 10−8 8 to 0.99 were acquired using a sorption analyzer ASAP 2020 (Micromeritics). Brunauer–Emmett–Teller equation was used to determine the specific surface area (SBET). From 5 to 10 adsorption points from 0.001 to 0.01 p/p0 were utilized to calculate SBET. Total pore volume was calculated on the basis of the nitrogen volume adsorbed at the relative pressure of 0.99. Micropore volume and pore size distribution were analyzed by DFT method.
The X-ray diffraction (XRD) patterns were collected on a X’Pert–PRO, Panalytical, Almelo X-ray diffractometer with 2 θ from 10 to 100°.
The field emission scanning electron microscopy (FE-SEM) images were taken by a SU8020 Ultra-High Resolution Field Emission Scanning Electron Microscope; Hitachi Ltd, under 5 kV voltage.
A sorption analyzer ASAP 2020 (Micromeritics) was applied to measure volumetric nitrogen and carbon dioxide uptake in a pressure range 0.02–1 bar, at a temperature of 0 and 20 °C.
Results and discussion
The nitrogen sorption isotherms investigated at a temperature of 77 K of activated carbons produced from avocado seeds using various liquids are presented in Fig. 1.
On the basis of the isotherm shape illustrating the amount of adsorbed gas at a particular specified pressure, it is possible to define the porosity characteristics of the adsorbent surface (Rashidi et al. 2016). Isotherms can be classified as type I (Sing 1985). As enhanced adsorption at relatively low pressure (less than 0.1 bar) is evident, suggesting that the resulting carbons have a well-developed microporous structure. Moreover, it is visible that a further part of the isotherm reached a plateau that is horizontally aligned with the axis of relative pressure, which suggests that in the structure prevails microporosity (Thommes et al. 2015). The isotherm of C_MeOH was the highest and started from about 400 cm3/g. That means that this activated carbon was the most microporous.
Table 1 compiles textural properties of activated carbons from avocado seeds. It was stated that all the materials were highly porous. The specific surface area ranged from 1464 to 2024 m2/g, depending on the liquid used. The highest value was observed for methanol. The highest pore volume, 0.9262 cm3/g, and micropore volume, 0.6813 cm3/g, were achieved for methanol, and the lowest data occurred for isopropanol (Vtot=0.631, Vmicro=0.506). The micropore content is very high for all the activated carbons. The highest micropore percentages were achieved for C_IzoOH. The values in Table 1 were in good agreement with Fig. 1.
Figure 2 presents the distribution of pore size calculated by the DFT method based on N2 adsorption measured at 77 K. All the activated carbon exhibited four sharp peaks at 0.50, 0.86, 1.19, and 1.60 nm. The first one is the sharpest and the highest, indicating high content of ultamicropores that play an essential role in CO2 adsorption (Deng et al. 2015; Serafin et al. 2017). C_H2O and C_IzoOH contained mostly micropores. For C_MeOH, mesopores in the 2–3 nm range were observed. Table 1 also showed that the C_MeOH contained more mesopores than the other activated carbons.
The carbon state of activated carbons was investigated by XRD method (Fig. 3). A very broad, deformed peak between 18 and 21° was observed. This signal can be attributed to the (002) surface of the turbostratic carbon (Wang et al. 2016). The peak of about 44° (100/101) was not visible, confirming the longitudinal dimension, the so-called aromatic sheets, of all the activated carbons were tiny, and the materials were primarily amorphous (Wu et al. 2018; Zhang et al. 2022a).
Figure 4 shows SEM images of activated carbons produced from avocado seeds using various liquids. All the materials were similar, with many cavities on the surface of the grains as a result of potassium hydroxide etching at the temperature of 850 °C. The system of well-organized macropores was observed.
Potassium hydroxide reacted with carbon, and the gases such as CO2, H2, and H2O were released, making pores in carbonaceous structures (Lillo-Ródenas et al. 2003):
KOH can also decompose according to:
The produced gases can also be involved in various reactions. For example (Yang et al. 2017a):
The produced potassium compounds, K2CO3, K2O, also could react with the char and influenced the activated carbon properties.
The textural properties allowed us to assume that activated carbons produced from avocado seed by this new method can be suitable CO2 sorbents. Table 2 shows the adsorption capacity of CO2 at a temperature of 0 °C (qCO2_0C) and 20 °C (qCO2_20C) and the adsorption capacity of N2 at a temperature of 20 °C (qN2_20C) at the pressure of 1 bar. Nitrogen adsorption was investigated in order to calculate the selectivity of CO2 adsorption over N2 in binary mixtures.
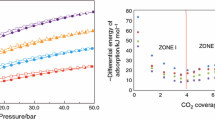
Figure 5 shows CO2 adsorption isotherms at the temperature of 0 °C. All the isotherms were similar. The highest CO2 adsorption at 1 bar and 0 °C (6.47 mmol/g) was achieved for activated carbons produced using methanol and isopropanol. When water was applied, adsorption was slightly lower, namely 6.31 mmol/g. CO2 adsorption at 1 bar, 0 °C on activated carbons produced from various sources is presented in Table 3. It is clearly seen that CO2 adsorption achieved by us using avocado seeds as a carbon source was very high compared to those presented by other researchers.
The kinetic diameter of CO2 molecules is 0.33 nm (D’Alessandro et al. 2010). The pores about two times larger than 0.33 nm are most favorable for CO2 adsorption. The adsorption potential affected by CO2 molecules from opposite walls in such micropores is the highest (Ghimire et al. 2019). All the samples were ultramicroporous, so the CO2 adsorption was so high.
Figure 6 presents CO2 and N2 adsorption isotherms at a temperature of 20 °C. The values of the CO2 adsorption at higher temperatures were lower but still high. The highest CO2 adsorption at 1 bar exhibited C_IzoOH (4.35 mmol/g), and the lowest adsorption (4.13 mmol/g) was observed over C_MeOH. The values of CO2 adsorption at 20 °C and 1 bar were strongly connected with microporosity, namely Vtot/Vmicro values. As was proved by others (Wickramaratne and Jaroniec 2013), CO2 adsorption on activated carbons at ambient conditions is strongly dependent on microporosity (Table 1). The decrease of the CO2 adsorption with the increase of the temperature indicated that physical adsorption took place.
Two two-parameter models (Freundlich and Langmuir) and two three-parameter models (Toth and Sips) were applied to analyze the experimental adsorption isotherms. The Freundlich , Langmuir, Toth, and Sips equations were described in Ayawei et al. (2017) and Serafin et al. (2023).
The applicability of the equations to fit the experimental data was established using the least-squares method. The lowest values of the errors was obtained using Sips model for CO2 and N2 adsorption. The Sips model is given by the equation:
where
- q:
-
The gas equilibrium adsorption at pressure p
- p:
-
Equilibrium pressure
- qm:
-
The saturation capacity
- b:
-
Equilibrium constant
- n:
-
Exponential parameter representing the heterogeneity of the material
The obtained parameters are presented in Table 4.
The saturation capacity decreased with the temperature increase, which confirmed the physical adsorption. The exponential parameters were close to one, proving the surface’s homogeneity. The values calculated by Sips equations are presented in Figs. 5 and 6 as lines, while points represented experimental data.
The N2 adsorption measurements were performed to calculate the CO2 adsorption selectivity over N2. The ideal adsorbed solution theory (IAST) (Myers and Prausnitz 1965) was applied to calculate the selectivity of carbon dioxide over nitrogen at 20oC. The selectivity of g1 over g2 is possible to calculate based on single adsorption isotherms of g1 and g1:
where
- xg1 (xg2):
-
The molar fractions of g1 (g2) gas in the adsorbed phase
- yg1, (yg2):
-
The molar fractions of g1 (g2) gas in the bulk phase
The accuracy of the IAST calculation was established for many gas mixtures on various sorbents (Herm et al. 2011; Lu et al. 2011).
Based on the IAST theory, the selectivity of CO2 adsorption for the equimolar mixture was calculated according to the equation:
The sorbents may be applied for CO2 removal from flue gas. The content of CO2 in off-gas depends on the kind of fossil fuels. If fuel gas is the energy source, the CO2 concentration is about 10%. For coal burning, CO2 concentration is equal to 15%. Taking into account the CO2 content in flue gas, the selectivity of CO2 over N2 for 10% and 15% CO2 content was also calculated.
- q i(p):
-
Adsorption value of i at pressure pi
For carbon dioxide, pCO2 were equal 0.1 and 0.15. For nitrogen, pN2 were equal to 0.9 and 0.85. In order to establish CO2 and N2 adsorption at a given pressure, the Sips model was applied.
The selectivity of CO2 adsorption for the equimolar mixture are presented in Fig. 7.
The highest CO2 adsorption was achieved over C_IzoOH activated carbon. Selectivity decreased with increasing pressure. The course of the curves was typical (Kiełbasa et al. 2022).
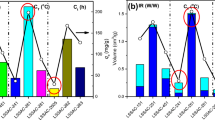
The selectivities of CO2 adsorption over N2 for typical flue gas concentrations 10 and 15% (SCO2_10, SCO2_15) are presented in Table 5. The highest selectivity was observed for C_IzoOH.
The reusability measurements of the best sorbent, C_Izo, are performed and presented in Fig. 8. Twenty CO2 adsorption/desorption cycles were performed at 0 °C and up to 1 bar for the best sorbent (C_Izo). As shown in Fig. 8, the CO2 adsorption capacity of this activated carbon was well maintained even after 20 cycles of adsorption/desorption processes. Intermediate cycles are not shown in the figure for clarity.
The Gibbs free energy change for the CO2 adsorption over C_Izo sample was calculated according to the equation (Tiwari et al. 2017):
where K is the equilibrium constant for CO2 adsorption
ΔHo and ΔSo can be obtained from the slope and intercept of van’t Hoff plot (Goel et al. 2015)
The negative values of Gibbs free energy change were obtained: − 1.03kJ/mol and − 0.56 kJ/mol for the temperature of 0 and 20 °C, respectively. Negative values indicated the feasibility and spontaneity of the adsorption process. Very small values of enthalpy and entropy were obtained: 0.08 J/mol and 0.03 kJ/(mol K). The negative value of enthalpy confirmed the exothermic nature of the adsorption. The positive value of entropy demonstrated the increase in randomness at the adsorbent-adsorbate interface and also adsorbent’s affinity towards carbon dioxide. Small value of ΔSo indicated that no signicant change in entropy took place.
Conclusions
In summary, we synthesized ultramicropore activated carbons from avocado seeds using combined solvothermal carbonization and thermal KOH activation method. The activated carbons with excellent textural properties (pore volume, micropore volume, and specific surface area) were successfully synthesized. All the samples were very good CO2 sorbents. The micropores were very important for CO2 adsorption. The highest CO2 adsorption and CO2 selectivity over N2 were achieved for activated carbon obtained using isopropanol that exhibited the highest micropore percentage making this material a promising candidate for carbon dioxide removal from mixtures of gasses. The highest CO2 adsorption at a pressure of 1 bar was achieved for C_Izo was equal to 6.47 mmol/g at 0 °C and 4.35 mmol/g at 20 °C. Because of the high CO2 adsorption, CO2 selectivity, and stability, this material can be applied for CO2 capture from flue gas.
Data availability
Not applicable.
References
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem 2017:1–11. https://doi.org/10.1155/2017/3039817
Bhaumik M, Choi HJ, Seopela MP et al (2014) Highly effective removal of toxic Cr(VI) from wastewater using sulfuric acid-modified avocado seed. Ind Eng Chem Res 53:1214–1224. https://doi.org/10.1021/ie402627d
Creamer AE, Gao B, Zhang M (2014) Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem Eng J 249:174–179. https://doi.org/10.1016/j.cej.2014.03.105
D’Alessandro DM, Smit B, Long JR (2010) Carbon dioxide capture: prospects for new materials. Angew Chemie Int Ed 49:6058–6082. https://doi.org/10.1002/anie.201000431
Deng S, Hu B, Chen T et al (2015) Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption 21:125–133. https://doi.org/10.1007/s10450-015-9655-y
Elizalde-González MP, Mattusch J, Peláez-Cid AA, Wennrich R (2007) Characterization of adsorbent materials prepared from avocado kernel seeds: natural, activated and carbonized forms. J Anal Appl Pyrolysis 78:185–193. https://doi.org/10.1016/j.jaap.2006.06.008
Ghimire PP, Dassanayake AC, Wickramaratne NP, Jaroniec M (2019) Polyvinyl pyrrolidone-assisted synthesis of size-tunable polymer spheres at elevated temperature and their conversion to nitrogen-containing carbon spheres. J Colloid Interface Sci 549:162–170. https://doi.org/10.1016/j.jcis.2019.04.059
Goel C, Bhunia H, Bajpai PK (2015) Resorcinol–formaldehyde based nanostructured carbons for CO 2 adsorption: kinetics, isotherm and thermodynamic studies. RSC Adv 5:93563–93578. https://doi.org/10.1039/C5RA16255F
He S, Chen G, Xiao H et al (2021) Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J Colloid Interface Sci 582:90–101. https://doi.org/10.1016/j.jcis.2020.08.021
Herm ZR, Swisher JA, Smit B et al (2011) Metal−organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture. J Am Chem Soc 133:5664–5667. https://doi.org/10.1021/ja111411q
Huang Y-F, Chiueh P-T, Lo S-L (2022) Carbon capture of biochar produced by microwave co-pyrolysis: adsorption capacity, kinetics, and benefits. Environ Sci Pollut Res 30:22211–22221. https://doi.org/10.1007/s11356-022-23734-x
Kiełbasa K, Bayar Ş, Varol EA et al (2022) Thermochemical conversion of lignocellulosic biomass - olive pomace - into activated biocarbon for CO2 adsorption. Ind Crops Prod 187:115416. https://doi.org/10.1016/j.indcrop.2022.115416
Kishibayev KK, Serafin J, Tokpayev RR et al (2021) Physical and chemical properties of activated carbon synthesized from plant wastes and shungite for CO2 capture. J Environ Chem Eng 9:106798. https://doi.org/10.1016/j.jece.2021.106798
Leite AB, Saucier C, Lima EC et al (2018) Activated carbons from avocado seed: optimisation and application for removal of several emerging organic compounds. Environ Sci Pollut Res 25:7647–7661. https://doi.org/10.1007/s11356-017-1105-9
Leite AJB, A CS, Thue PS et al (2017) Activated carbon from avocado seeds for the removal of phenolic compounds from aqueous solutions. Desalin Water Treat 71:168–181. https://doi.org/10.5004/dwt.2017.20540
Lillo-Ródenas M, Cazorla-Amorós D, Linares-Solano A (2003) Understanding chemical reactions between carbons and NaOH and KOH. Carbon N Y 41:267–275. https://doi.org/10.1016/S0008-6223(02)00279-8
Lu W, Yuan D, Sculley J et al (2011) Sulfonate-grafted porous polymer networks for preferential CO 2 adsorption at low pressure. J Am Chem Soc 133:18126–18129. https://doi.org/10.1021/ja2087773
Ma C, Lu T, Shao J et al (2022) Biomass derived nitrogen and sulfur co-doped porous carbons for efficient CO2 adsorption. Sep Purif Technol 281:119899. https://doi.org/10.1016/j.seppur.2021.119899
Myers AL, Prausnitz JM (1965) Thermodynamics of mixed-gas adsorption. AIChE J 11:121–127. https://doi.org/10.1002/aic.690110125
Parshetti GK, Chowdhury S, Balasubramanian R (2015) Biomass derived low-cost microporous adsorbents for efficient CO 2 capture. Fuel 148:246–254. https://doi.org/10.1016/j.fuel.2015.01.032
Rashidi NA, Yusup S, Borhan A (2016) Isotherm and thermodynamic analysis of carbon dioxide on activated carbon. Procedia Eng 148:630–637. https://doi.org/10.1016/j.proeng.2016.06.527
Rodrigues LA, da Silva MLCP, Alvarez-Mendes MO et al (2011) Phenol removal from aqueous solution by activated carbon produced from avocado kernel seeds. Chem Eng J 174:49–57. https://doi.org/10.1016/j.cej.2011.08.027
Salomón-Negrete MÁ, Reynel-Ávila HE, Mendoza-Castillo DI et al (2018) Water defluoridation with avocado-based adsorbents: synthesis, physicochemical characterization and thermodynamic studies. J Mol Liq 254:188–197. https://doi.org/10.1016/j.molliq.2018.01.084
Serafin J, Dziejarski B, Cruz Junior OF, Sreńscek-Nazzal J (2023) Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon N Y 201:633–647. https://doi.org/10.1016/j.carbon.2022.09.013
Serafin J, Narkiewicz U, Morawski AW et al (2017) Highly microporous activated carbons from biomass for CO 2 capture and effective micropores at different conditions. J CO2 Util 18:73–79. https://doi.org/10.1016/j.jcou.2017.01.006
Serafin J, Ouzzine M, Cruz Junior OF, Sreńscek-Nazzal J (2021) Preparation of low-cost activated carbons from amazonian nutshells for CO2 storage. Biomass and Bioenergy 144:105925. https://doi.org/10.1016/j.biombioe.2020.105925
Serafin J, Ouzzine M, Xing C et al (2022) Activated carbons from the Amazonian biomass andiroba shells applied as a CO2 adsorbent and a cheap semiconductor material. J CO2 Util 62:102071. https://doi.org/10.1016/j.jcou.2022.102071
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198557040603
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Tiwari D, Bhunia H, Bajpai PK (2017) Epoxy based oxygen enriched porous carbons for CO 2 capture. Appl Surf Sci 414:380–389. https://doi.org/10.1016/j.apsusc.2017.04.128
Wang D, Liu S, Fang G et al (2016) From trash to treasure: direct transformation of onion husks into three-dimensional interconnected porous carbon frameworks for high-performance supercapacitors in organic electrolyte. Electrochim Acta 216:405–411. https://doi.org/10.1016/j.electacta.2016.09.053
Wickramaratne NP, Jaroniec M (2013) Activated carbon spheres for CO 2 adsorption. ACS Appl Mater Interfaces 5:1849–1855. https://doi.org/10.1021/am400112m
Wu Z, Tian K, Huang T et al (2018) Hierarchically porous carbons derived from biomasses with excellent microwave absorption performance. ACS Appl Mater Interfaces 10:11108–11115. https://doi.org/10.1021/acsami.7b17264
Yang HM, Zhang DH, Chen Y et al (2017a) Study on the application of KOH to produce activated carbon to realize the utilization of distiller’s grains. IOP Conf Ser Earth Environ Sci 69:012051. https://doi.org/10.1088/1755-1315/69/1/012051
Yang J, Yue L, Hu X et al (2017b) Efficient CO 2 capture by porous carbons derived from coconut shell. Energy & Fuels 31:4287–4293. https://doi.org/10.1021/acs.energyfuels.7b00633
Yang Z, Zhang G, Xu Y, Zhao P (2019) One step N-doping and activation of biomass carbon at low temperature through NaNH2: an effective approach to CO2 adsorbents. J CO2 Util 33:320–329. https://doi.org/10.1016/j.jcou.2019.06.021
Zhang J, Yin R, Shao Q et al (2019) Oxygen vacancies in amorphous InO x nanoribbons enhance CO 2 adsorption and activation for CO 2 electroreduction. Angew Chemie - Int Ed 58:5609–5613. https://doi.org/10.1002/anie.201900167
Zhang R, Qiao J, Zhang X et al (2022a) Biomass-derived porous carbon for microwave absorption. Mater Chem Phys 289:126437. https://doi.org/10.1016/j.matchemphys.2022.126437
Zhang Y, Wei Z, Liu X et al (2022b) Synthesis of palm sheath derived-porous carbon for selective CO 2 adsorption. RSC Adv 12:8592–8599. https://doi.org/10.1039/D2RA00139J
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Joanna Siemak. The first draft of the manuscript was written by Joanna Siemak. Beata Michalkiewicz supervised the work. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors agree to publish this work in ESPR.
Competing interests
The authors declare no competing interests
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siemak, J., Michalkiewicz, B. Enhancement of CO2 adsorption on activated carbons produced from avocado seeds by combined solvothermal carbonization and thermal KOH activation. Environ Sci Pollut Res (2023). https://doi.org/10.1007/s11356-023-28638-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-023-28638-y