Abstract
Phytoremediation is considered an effective method for indoor air pollution control. The removal rate and mechanism of benzene in air by two plants, Tradescantia zebrina Bosse and Epipremnum aureum (Linden ex André) G. S. Bunting, were investigated through fumigation experiments under the condition of plant hydroponics culturing. Results showed that the plant removal rates increased with increase in benzene concentration in air. When the benzene concentration in air was set at 432.25–1314.75 mg·m−3, the removal rates of T. zebrina and E. aureum ranged from 23.05 ± 3.07 to 57.42 ± 8.28 mg·kg–1·h–1 FW and from 18.82 ± 3.73 to 101.58 ± 21.20 mg·kg–1·h–1 FW, respectively. The removal capacity was positively related to the transpiration rate of plants, indicating that gas exchange rate could be a key factor for the evaluation of removal capacity. There existed fast reversible transport of benzene on air-shoot interface and root-solution interface. After shoot exposure to benzene for 1 h, downward transport was the dominant mechanism in the removal of benzene in air by T. zebrina, while in vivo fixation was the dominant mechanism at exposure time of 3 and 8 h. Within 1–8 h of shoot exposure time, in vivo fixation capacity was always the key factor affecting the removal rate of benzene in the air by E. aureum. Contribution ratio of in vivo fixation in the total benzene removal rate increased from 6.29 to 92.29% for T. zebrina and from 73.22 to 98.42% for E. aureum in the experimental conditions. Reactive oxygen species (ROS) burst induced by benzene exposure was responsible for the contribution ratio change of different mechanisms in the total removal rate, which also was verified by the change of activities of antioxidant enzymes (CAT, POD, and SOD). Transpiration rate and antioxidant enzyme activity could be considered parameters to evaluate the plant removal ability to benzene and to screen plants for establishment of plant-microbe combination technology.
Similar content being viewed by others
Introduction
Indoor air pollution resulting from our daily life and work becomes a global environmental health problem (Baloch et al. 2020). It was reported that about 4% of global diseases were related to indoor environmental pollution, and that nearly 3 million deaths in developing countries probably have been caused by indoor air pollution since the beginning of the new century (González-Martín et al. 2021). Building ventilation is significantly restricted by the building energy conservation and extreme outdoor temperature conditions and exacerbated indoor air problems. In an airtight indoor environment designed for energy-saving purposes, harmful compounds emitted from decoration and refurbishing materials tend to accumulate owing to the low rate of air exchange. Benzene is also an indoor environmental toxicant which comes from furniture painting, wall painting, and decoration materials (Isinkaralar 2022). The concentration of benzene in the indoor air in the kitchen or industrial site may exceed the national standard by tens or even hundreds of times (Natarajan et al. 2022). In recent years, people have spent much time indoors due to the wide spread of the COVID-19 virus, and the health risks caused by indoor air pollution are increasing (Ptbsb et al. 2020). More and more investigations have been focused on the health risk and elimination of indoor air pollution.
Volatile organic compounds (VOCs) are common indoor air pollutants that can be effectively removed by physical, chemical, and biological methods (Lhab et al. 2021; Liu et al. 2021a; Yue et al. 2020). At present, indoor air is usually purified by commercial purifiers based on physical adsorption or catalytic oxidation mechanisms. These purifiers may result in energy consumption, secondary pollution, or high investment problem. Biotechnology is also developing for the indoor air pollution control (Lu et al. 2010), including microbiological method, phytoremediation, and microbe and plant combined remediation. Phytoremediation technology is widely regarded as an important means to control indoor air pollution due to safety, economy, and beauty (Chen et al. 2020; Kurade et al. 2021).
Many ornamental plants can efficiently remove VOCs in the air (Brilli et al. 2018; Wei et al. 2020). The phytoremediation process of indoor air pollutants can be divided into three steps. First, VOC molecules, which diffuse to plants, can be fixed on the leaves surface by crude wax and then enter the interior of shoot through passive diffusion or active absorption (Redondo-Bermúdez et al. 2021; Treesubsuntorn et al. 2013). Second, VOC molecules can be accumulated in plant tissues and degraded by plant metabolism as well as downward transported to the root through the phloem (Han et al. 2021). Physiological indexes such as chlorophyll content, relative permeability of plasma membrane, and peroxidase content will change when plants experience a stress condition (Teiri et al. 2018). Finally, VOCs are released into the rhizosphere environment by plant root and assimilated by soil microorganisms symbiotically living with the plant. The synergistic effect of plants and microorganisms could improve the removal capacity of plants for VOCs (Abhilash et al. 2011; Yang et al. 2020). Thus, there are three key aspects controlled by plants in implementation process of phytoremediation to airborne benzene, including diffusion and shoot uptake and in vivo fixation as well as downward transport and root release.
Formaldehyde, VOCs, and particulate matter were the main indoor air pollutants. Among them, VOCs have been recognized as one of the principal trace constituents of indoor air pollutants (González-Martín et al. 2021). As a typical VOC, the benzene in indoor air should be control because of its high toxicity and high content. Indoor air quality depends on outdoor air quality and indoor pollutant sources. For example, when the outdoor air pollution is serious, it will have a negative impact on the indoor air quality. Smoking, cooking, solvents, and coating mediums for furniture as well as room decorations are potential indoor emission sources of benzene (González-Martín et al. 2021). The released process of benzene may last for several years or decades (Li et al. 2014; Liu et al. 2020). Pollutants such as benzene still widely exist in indoor environment. Long-term exposure to airborne benzene may lead to serious health risks such as respiratory and nervous system diseases and even organ failure. Therefore, benzene is called as one of the “three invisible killers” (Dimitriou and Kassomenos 2020; Ir et al. 2021; Ren et al. 2020).
At present, most studies on the phytoremediation to airborne benzene are mainly focused on potted plants. With this culture pattern, the measured removal efficiency was a comprehensive index to evaluate the total purification ability of the plant-soil system. It cannot separately define the contribution of soil, plants, and microorganisms to the total removal efficiency. Thus, pot cultivation pattern was not conducive to the quantitative exploration of plant purification mechanism. Hydroponic culture can simplify the experimental conditions and conductive to further explore the mechanism of phytoremediation. Many plants have been proved to have strong ability to remove benzene (Parseh et al. 2018). However, it is still difficult to predict and ensure the phytoremediation efficiency under different environmental conditions due to the lack of effective parameters. The lack of key evaluation factors on phytoremediation also limits the capture of efficient purification plants and the development of highly efficient combined bioremediation technology (Mosaddegh et al. 2014; Paull et al. 2019). These deficiencies limit the engineering application of phytoremediation technology in airborne benzene pollution. It is necessary to identify key parameters in the process of benzene removal by plants.
Thus, the aims of this study are to (i) investigate the reversible transport of benzene in air-plant-water system, (ii) find the key factors affecting plant purification capacities, and (iii) quantitatively divide the removal mechanisms of airborne benzene by plants and contribution ratio change of different mechanisms in the processes of benzene removal.
Materials and methods
Chemicals
Benzene and chemicals used for plant culturing were all of analytical grade.
Preparation of plant seedlings
Seedlings of uniform-sized T. zebrina and E. aureum were separated from their mother plant and rinsed with deionized water. Then, the seedlings were transplanted into a 4-L container filled with a sterilized 1/5-strength Hoagland nutrient. Seedlings were grown hydroponically for 4 weeks in a growth chamber before experimentation. The temperature of the control environment was 23–25 °C and the humidity was 70%. The nutrient solution for seedlings was aerated continuously and refreshed every 2 days. The composition of the Hoagland nutrient solution was: 2.5 mM KNO3, 2.5 mM Ca(NO3)2, 0.5 mM MgSO4, 0.1 mM KH2PO4, 1.0 μM MnCl2, 3 μM H3BO3, 1 μM (NH4)6Mo7O24, 1 μM ZnSO4, 0.2 μM CuSO4, and 60 μM Fe(III)-ethylenediaminetetra-acetic acid (EDTA). The pH of the nutrient solution was adjusted to 6.0 using 0.1 M KOH or HCl solution.
Treatments with benzene
For a certain plant species, plants with the same number of leaves and similar fresh weight were selected. A total of 54 plants were used in the experiments. The test plants from one set of experiments were not re-employed in the other experiments. The roots were rinsed with deionized water and wiped dry. The fresh weight of plant seedlings was measured. Subsequently, the seedlings were transplanted to glass bottles containing 10 mL of Hoagland nutrient solution before the benzene exposure experiment (one plant per bottle). The glass bottles were wrapped with aluminum foil paper, and the open area between the bottle and the stem of the plant was sealed with a sponge wrapped with aluminum foil paper. The nutrient solution was sterilized and continuously aerated for 1 h before it was used in the experiment.
During the experiments, three glass bottles with plants and one glass bottle without plants (control A) were carefully placed into a transparent glass container; at the same time, avoid plants and carefully put the perforated ampoule bottle containing benzene liquid into the container. Subsequently, the container was immediately sealed. Details of the experimental setup are shown in Fig. 1. A control without plants in the glass container with four glass bottles (control B) was used to test the loss of benzene caused by photochemical reaction, gas leakage, and other processes. The initial benzene contents in air were set at three levels (C1: 432.25 mg·m−3, C2: 876.50 mg·m−3, and C3: 1314.75 mg·m−3) with triplicate samples for each level. The shoot exposure time was set at 1, 3, and 8 h (Table 1).
At each set time, the benzene contents in the container were measured. Immediately, the plants were taken out from the container, and the glass bottle with plant seedlings was quickly weighed. Rhizosphere solution in bottles with or without plants from three replicated was collected for determination of the benzene concentration.
After shoot exposure to benzene, the seedling roots were washed with deionized water and then transferred to glass bottles containing fresh Hoagland nutrient solution. Subsequently, the seedlings were quickly put into a new transparent glass pot without benzene. The benzene concentration in the air of the pot was measured every 30 min.
Analysis of benzene concentrations
Before and after shoot exposure, the level of benzene in the air of glass container and rhizosphere solutions was measured by headspace gas chromatography. Benzene level in the air of the container was sampled and analyzed by a device with a glass syringe, a three-way valve, and gas chromatograph (as shown in Fig. 1).
Measurement of plant transpiration rate
Plants seedlings with similar biomass and the same leave numbers were selected. The roots were rinsed with deionized water and quickly wiped dry. The fresh weight of plant seedlings was measured. Subsequently, the seedlings were transplanted to glass bottles containing 10 mL of Hoagland nutrient solution before the benzene exposure experiment (one plant per bottle). The glass bottles were wrapped with aluminum foil paper, and the open area between the bottle and the stem of the plant was sealed with a sponge wrapped with aluminum foil paper. We reduce the open space to prevent water from evaporating directly from the nutrient solution to the outside air. Seedlings, aluminum foil, sponges, nutrient solution, and glass bottle are weighed together. Then, we put the glass bottles with plants into containers with or without benzene for fumigation treatment. At the set times (1, 3, and 8 h), the glass bottle containing plants was removed, and the total weight of the glass bottle with seedlings was measured. The weight of water lost by transpiration can be accurately determined by comparing the weight changes before and after exposure.
Analysis of CAT, POD, and SOD activities
The determination methods for catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) activities were according to the previous report (Su and Liang 2015).
Analysis of ROS level in plant leaves
Samples of plant leaves were extracted with 2 mL of phosphate buffer (pH = 7.8) for three times. The extracted solutions were combined and centrifuged, and 0.5 mL clear liquids were separated from extracts, 0.5 mL phenanthroline solution was added, and then 0 or 0.5 mL FeSO4 solution (1.865 mmol·L−1) was added. After dilution (and mixing) with distilled water to 10 mL, the mixtures were put into a thermostated water bath fixed at 37 ± 2 °C for 1 h, cooled with cold water, and then filtered. The liquid phase was collected and diluted, and then the absorbance at 536 nm was measured using a UV-Vis spectrophotometer. Two controls were set, one with no leaf extract added and the other without FeSO4 added. The differences in absorbance between mixture solution and two controls were used to express the oxidizing capability of the plant leaf extract. For quantitatively calculating the oxidizing capability, varied volumes of 0.01% (v/v) H2O2 solution and 0.5 mL of phenanthroline solution were added into a series of 0.5 mL FeSO4 solution. The curve for the absorbance change with the amount of H2O2 was drawn to estimate the oxidizing capability of plant leaves (or the ROS level in plant tissues).
Chemical analyses
The concentration of benzene was measured by a gas chromatograph (GC7980; Techcomp, China) equipped with hydrogen flame ionization detector (FID) using a TM-5 capillary column (0.53 mm × 30 m × 1 μm). N2 was generated with a nitrogen generator (Beijing Purkinje General Instrument, Beijing, China) as the carrier gas at a flow rate of 15 mL/min. The injector port was maintained at 160 °C, and a 1.0 mL volume of gas was injected in split mode (split ratio 18:1). The column temperature was set at 50 °C. The detector temperature was maintained at 180 °C.
Data analysis
SPSS 21.0 was used for one-way analysis of variance.
Results and discussion
Biomass
The fresh weight of T. zebrina and E. aureum ranged from 3.45 to 4.31 g and from 4.14 to 5.92 g, respectively. The leaf areas of each plant of T. zebrina and E. aureum were 120.75 to170.80 cm2 and 29.00 to 39.85 cm2.
Benzene removal rate of plants
There was no visible change in the leaf color, area, and shape of T. zebrina and E. aureum after shoot exposure to benzene. That indicated the strong tolerance of the two plants to benzene during the experimental period (Fig. 2).
The loss range of benzene in the container of control B was 1.21–3.22%. The benzene in the container containing T. zebrina and E. aureum was reduced by 40.58 and 55.88%, respectively (the loss in control B was deducted). Results showed that two species of plants could effectively remove benzene from the air. There was a chemical potential difference of benzene between the plant tissues and air, which enabled the benzene to be absorbed by the plants by diffusion (Liu et al. 2021b). The removal rate of benzene by plants was expressed based on the difference in benzene content of containers before and after plant exposure treatment, plant fresh weight, and exposure time. Under initial benzene concentration of 432.25–1314.75 mg·m−3, the removal rate of T. zebrina and E. aureum ranged from 23.05 ± 3.07 to 91.84 ± 7.97 mg·kg−1·h−1 FW and 18.82 ± 3.73 to 159.03 ± 9.07 mg·kg−1·h−1FW, respectively (Table 2). In previous studies, common indoor ornamental plants such as Chlorophytum comosum Baker, Syngonium podophyllum Schott, and E. aureum could remove about1.97–2.65 mg·m−2·h−1 of benzene (Sriprapat et al. 2016).
The concentration of benzene in the air was one of the important factors affecting the removal rate. For T. zebrina, the removal rate was not related to the concentration of benzene in the air at exposure time of 1 h, while the positive relationship of plant removal rate and initial benzene concentration in air was found at the shoot exposure time of 3 or 8 h. For E. aureum, the removal rate was always positively related to the initial benzene concentration in air at setting exposure time. The shoot exposure time was another important influence factor on benzene removal by plants. The removal rate decreased gradually with increase in exposure time due to the decrease in benzene level in air during experiment period (Table 2).
Plant transpirations
The stomata on the surface of leaves provide the primary channels for gas exchange and transpiration in the process of photosynthesis; stomatal oscillation will have an immediate impact on the capacity of gas exchange and the rate of transpiration for plants (Pérez et al. 2021, Treesubsuntorn et al. 2013). In this research, transpiration rate was used to evaluate the active absorption of benzene by plants from air.
Results showed that the plant transpiration rates decreased with the increase in benzene concentration and exposure time (Fig. 3). After shoot exposure to benzene for 1 h, the transpiration rates of T. zebrina and E. aureum dropped rapidly from 50.51 ± 3.57 to 26.80 ± 2.01 g·kg−1·h−1 FW and from 17.43 ± 3.49 to 9.56 ± 0.91 g·kg−1·h−1 FW, respectively, with the shoot exposure concentration changing from 432.25 to 1314.75 mg·m−3. Transpiration rate of plants sharply decreased with the increase in exposure time. After shoot exposure to the air with 432.25 mg·m−3 benzene for 8 h, about 37 and 89% reduction in the transpiration rates was found in T. zebrina and E. aureum, respectively (Fig. 3). Results showed that transpiration rate was positively related to removal capacity of plants (Fig. 3). The gas exchange was a key factor in airborne benzene removal; the transpiration rate could be considered one of parameters to evaluate the plant ability to remove benzene.
Time-dependent curves of benzene transpiration rates by Tradescantia zebrina Bosse (a) and Epipremnum aureum (Linden ex André) G. S. Bunting (b) under the condition of shoot exposure to benzene at 432.25 mg·m−3 (C1), 876.50 mg·m−3 (C2), and 1314.75 mg·m−3 (C3). Each point was the mean of three replicates. Error bars represented SE
Reversible transport of benzene by plants
Reversible transfer of benzene in the air-plant interface
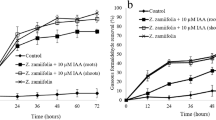
After shoot exposure to 432.25 mg·m−3 benzene in air for setting times (1, 3, and 8 h), plant was taken out; we washed the root with deionized water, wrapped it dry and then put it into a new bottle with fresh nutrient solution. Bottles with plants were quickly transferred into a benzene-free container to investigate the benzene release process in the plant-air system. Results proved that there existed a rapidly reversible interfacial transfer of benzene between plant leaves and air. The concentration of benzene in the air of the container increased rapidly during the first 0.5–2 h and then gradually stabilized (Fig. 4). When the contaminated plant shoots were exposed to a clean air, benzene that accumulated in plant tissues was released into the air. This result was consistent with the previous report on the shoot release of formaldehyde by Chlorophytum comosum seedlings (Su and Liang 2015). There are great differences in benzene concentration on air-plant interface in the initial stage, which lead to fast diffusion of benzene from shoot to air. Limited accumulation of benzene in shoot restricted the release capacity and rate of shoot release, which led to the decline in the release rate with increase in release time.
Environmental factors and the plant characteristics may influence the shoot uptake and release of benzene. After shoot exposure to 432.25 mg·m−3 benzene in air for 1 h, the shoot release capacity of T. zebrina was obviously higher than that of E. aureum. Nevertheless, the shoot release capacity of E. aureum was higher than that of T. zebrina after shoot exposure to the air with 432.25 mg·m−3 benzene for 3 or 8 h. Related bodies of literature illustrate that the characteristics of villi and rough leaf surface in plant make it easy for contaminants to adhere (Lee et al. 2020). The leaf surface of T. zebrina was villi and rough, while the leaf surface of E. aureum was smooth. Therefore, the adsorption capacity of T. zebrina to benzene was greater than that of E. aureum, which leads to the release capacity of T. zebrina to benzene being greater than that of E. aureum after 1-h benzene exposure. The similar phenomenon was found in the previous investigation on Pinus tabuliformis (Lu et al. 2018). The shoot release capacity and rate of T. zebrina to benzene increased gradually with the increase in shoot uptake time in the range of 0–3 h (Fig. 4a). To the E. aureum, an inverse relationship between shoot exposure time and shoot release capacity was found (Fig. 4b). The release capacity of benzene from T. zebrina increased with increase in exposure time, while the release capacity of benzene by E. aureum decreased with the increase in shoot exposure time. There were interspecific differences in the ability and principle of uptake and release of benzene between T. zebrina and E. aureum. The downward transport capacity of benzene by T. zebrina and E. aureum was approximated, while the in vivo fixation capacity of E. aureum was significantly stronger than that of T. zebrina. Benzene stress for a long time led to the gradual enhancement of stress response of E. aureum, thus enhancing the in vivo fixation ability. Therefore, the release capacity decreased with the increase in exposure time.
Transfer of benzene in root-solution interface
Benzene in leaves could be downward transported partially from shoots to roots via phloem and then released into the rhizosphere solution (Fig. 5). The concentration of benzene in the rhizosphere solution was related to the shoot exposure concentration and exposure time, which was consistent with the previous report on formaldehyde (Liang et al. 2019). The benzene concentration in bottle solutions collected from control A was below the detection limit when the content of benzene in the air was set to 432.25–1314.75 mg·m−3. Nevertheless, benzene in two plant rhizosphere solutions could be detected after shoot exposure for the setting times. Downward transport rate of benzene from air to rhizosphere was calculated by the concentration of benzene in rhizosphere solution and the fresh weight of plants. The calculation results are shown in Fig. 5. According to the literature reports, benzene in rhizosphere solution could be quickly taken up, upward transported via xylem to shoot, and then volatilized into the air (Su and Liang 2015). Therefore, there existed a rapidly reversible interfacial transfer of benzene between plant roots and rhizosphere solution.
Downward transport rates of benzene from air to rhizosphere solution by Tradescantia zebrina Bosse (a) and Epipremnum aureum (Linden ex André) G. S. Bunting (b) at the benzene concentration in air of 432.25 mg·m−3 (C1), 876.50 mg·m−3 (C2), and 1314.75 mg·m−3 (C3). Each point was the mean of three replicates. Error bars represented SE
In the time-dependent experiments, the detected downward transport rate could reach up to 38.82 ± 12.17 mg·kg−1·h−1 FW and 37.23 ± 8.74 mg·kg−1·h−1 FW for the T. zebrina and E. aureum, respectively. Results indicated the shoot uptake and downward transport of benzene were a rapid process. The transmission between different interfaces in air-plant-solution systems was the key influence factor in plant removal rate of benzene in air within 1 h exposure time. The plant downward transport rate of benzene by plants declined with increase in shoot exposure time (Fig. 5). As shoot exposure time was set at 3 and 8 h, the downward transport rate of T. zebrina was 13.85 ± 2.94 and 2.67 ± 0.19 mg·kg−1·h−1 FW, while that of E. aureum was 4.21 ± 0.47 and 3.89 ± 1.34 mg·kg−1·h−1 FW. Two aspect factors are responsible for the above results, the continuous decrease of benzene in container air due to the phytoremediation and the constant increase in the in vivo purification capability induced by rising reactive oxygen species (ROS) level.
In the concentration-dependent experiment, the downward transport rate of two plants increased with increase in benzene concentration in air (Fig. 5). These results were in line with previous reports on the shoot uptake and transport of trichloroethylene and 1,2,3-trichlorobenzene by wheat, corn, and tomatoes (Su and Liang 2013).
Antioxidant enzyme activity in plant extracts
Previous studies have shown that abiotic stresses usually destroy cell structure by the production of high levels of reactive oxygen species (ROS), affecting plant growth and even causing plant death (Liang et al. 2019; Gong et al. 2019). Plants have developed a variety of defense strategies such as expression of antioxidase to adapt to detrimental conditions. Catalases (CAT), peroxidase (POD), and superoxide dismutase (SOD) are important antioxidant enzymes in plant tissue and constitute a primary source of cellular defenses against cell damages by ROS and can catalyze H2O2 to produce H2O and O2 (Hippeli and Elstner 1996). The activities of three antioxidant enzymes in T. zebrina and E. aureum have been tested before and after shoot exposure to benzene. The activities of the three antioxidant enzymes (CAT, POD, and SOD) are listed in Table 3. The activity of CAT and POD increased with the increase in benzene concentration in air and exposure time, and that CAT and POD were more sensitive than SOD to benzene stress. The process of “redox regulation” protects living organisms from various oxidative stresses and maintains “redox homeostasis” by controlling the redox status in vivo (Linghu et al. 2011). It corresponded to the enhancement of active oxygen scavenging ability of CAT and POD. High antioxidant enzyme activities usually reflect high ROS levels in plants or high resistance ability of plants under adversity stress (Khaksar et al. 2017). The rapid increase in CAT and POD activities corresponds to a large number of outbreaks of ROS and high in vivo degradation ability of plants. It can be inferred that the change in antioxidant enzyme activity corresponded to the change in contribution rate of in vivo degradation mechanism. At the same time, the increase in antioxidant enzyme activity indicates that plants have stronger stress resistance after benzene stress. The plant ROS level in benzene treatment was obviously higher than that in the control, which was also the evidence of above conclusion.
Contribution ratio of downward transport and in vivo fixation mechanisms
The plant removal of benzene in air could be roughly divided into downward transport and in vivo fixation. The contribution ratio of in vivo fixation mechanism is calculated by mass balance shown in Fig. 6. As exposure time was set at 1 h, the downward transport via phloem was the dominant mechanism with a contribution ratio of 93.71% on the benzene removal by T. zebrina from air. The contribution ratio of downward transport mechanism decreased with increase in shoot exposure time. The contrary regulation was found in the relationship of the contribution ratio of in vivo fixation mechanism and shoot exposure time. The in vivo fixation mechanism has absolutely dominated the plant removal of benzene in air when shoot exposure time was set at 3 and 8 h. The highest contribution ratio of fixation mechanism reached up to 92.29 and 98.42% for T. zebrina and E. aureum, respectively (Fig. 6). The contribution ratio of in vivo fixation mechanism increased from 6.29 to 92.29% for T. zebrina and from 73.22 to 98.42% for E. aureum under the experimental conditions. At the beginning of the shoot uptake, the high chemical potential difference in different interfaces of air-plant-solution caused the high transport rate of benzene from air to rhizosphere solution. As shoot exposure time increased, ROS burst caused the sharp rise in the dissipation ability to accumulate benzene in plant tissue (Oksanen and Kontunen-Soppela 2021), resulting in the fast increase in contribution ratio of the in vivo fixation mechanism.
Oxidation potential of leaf extracts
In order to further discuss the phytoremediation mechanism of plants with benzene in air, the oxidation potentials of leaf extracts derived from T. zebrina and E. aureum were investigated. The oxidation potential of a leaf extract was expressed as the equivalent H2O2 content in samples needed to oxidize the added Fe2+ in this experiment (Fig. 7). The observed equivalent H2O2 content in two plant control samples (the leaf extract of untreated plants) was 318.32 ± 3.35 and 225.51 ± 11.60 μmol H2O2·g−1 FW, corresponding to T. zebrina and E. aureum. After shoot exposure to 432.25 mg·m−3 benzene in air for 3 h, the observed equivalent H2O2 content in T. zebrina and E. aureum was 345.35 ± 25.27 and 292.76 ± 2.19 μmol H2O2·g−1 FW, respectively. Compared with the plants without benzene exposure, the plants with benzene exposure had higher oxidation potential, which corresponded to the in vivo fixation ability of plants to benzene and the activity of antioxidant enzymes. It can support the view that ROS regulate the contribution rate of plant purification mechanism.
Conclusion
Benzene in the air was effectively removed by T. zebrina and E. aureum. The removal rate of T. zebrina and E. aureum ranged from 23.05 ± 3.07 to 57.42 ± 8.28 mg·h−1·kg−1 FW and from 18.82 ± 3.73 to 101.58 ± 21.20 mg·h−1·kg−1 FW, respectively. Benzene could be reversibly transported rapidly at the air-plant and root-rhizosphere solution interfaces in the air-plant-solution system. Removal and transport rates were negatively correlated with exposure time but positively correlated with benzene concentration in air. ROS burst induced by benzene and adjusted by the antioxidant enzyme activities in plant caused the change in dominant removal mechanism of plants. Many factors including benzene concentration, exposure time, transpiration rate, and antioxidant enzyme activity could affect the ability of plants to remove airborne benzene, among which transpiration rate and antioxidant enzyme activity were the most important influence factors. Transpiration rate and antioxidant enzyme activity could be considered the key parameters to evaluate the benzene removal ability of plants. The research could be used to roughly predict the purification ability of plants to VOCs and provide a methodological basis for the screening and culture technology of plants with high VOC removal ability. It could be also used to develop plant-microbial combined technology.
References
Abhilash PC, Srivastava S, Srivastava P, Singh B, Jafri A, Singh N (2011) Influence of rhizospheric microbial inoculation and tolerant plant species on the rhizoremediation of lindane. Environ Exp Bot 74:127–130
Baloch RM, Maesano CN, Christoffersen J, Banerjee S, Dewolf MC (2020) Indoor air pollution, physical and comfort parameters related to school children’s health: data from the European sinphonie study. Sci Total Environ 739:139870
Brilli F, Fares S, Ghirardo A, Visser PD, Menghini F (2018) Plants for sustainable improvement of indoor air quality. Trends Plant Sci 23:507–512
Chen RY, Ho KF, Hong GB, Chuang KJ (2020) House plant, indoor air pollution, and cardiovascular effects among elderly subjects in Taipei, Taiwan. Sci Total Environ 705(25):135770
Dimitriou K, Kassomenos P (2020) Background concentrations of benzene, potential long range transport influences and corresponding cancer risk in four cities of central Europe, in relation to air mass origination. J Environ Manage 262:110374
Gong S, Ding Y, Hu S, Ding L, Chen Z, Zhu C (2019) The role of HD-Zip class I transcription factors in plant response to abiotic stresses. Physiol Plant 167(4):516–525
González-Martín J, Kraakman N, Pérez C, Lebrero R, Muoz R (2021) A state-of-the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 262:128376
Han Y, Lee J, Haiping G, Kim K-H, Wanxi P, Bhardwaj N, Oh J-M, Brown RJC (2021) Plant-based remediation of air pollution: a review. J Environ Manage 301:113860
Hippeli S, Elstner EF (1996) Mechanisms of oxygen activation during plant stress: biochemical effects of air pollutants. J Plant Physiol 148(3):249–257
Huang L, Wei Y, Zhang L, Ma Z, Zhao W (2021) Estimates of emission strengths of 43 VOCs in wintertime residential indoor environments, Beijing. Sci Total Environ 793:148623
Ir A, Sdm A, Pcsa B, Plz A (2021) Benzene exposure and non-Hodgkin lymphoma: a systematic review and meta-analysis of human studies. Lancet Planet Health 5:633–643
Isinkaralar K (2022) High-efficiency removal of benzene vapor using activated carbon from Althaea officinalis L. biomass as a lignocellulosic precursor. Environ Sci Pollut R 29(44):66728–66740
Khaksar G, Treesubsuntorn C, Thiravetyan P (2017) Effect of exogenous methyl jasmonate on airborne benzene removal by Zamioculcas zamiifolia: the role of cytochrome P450 expression, salicylic acid, IAA, ROS and antioxidant activity. Environ Exp Bot 138:130–138
Kurade MB, Ha Y-H, Xiong J-Q, Govindwar SP, Jang M, Jeon B-H (2021) Phytoremediation as a green biotechnology tool for emerging environmental pollution: a step forward towards sustainable rehabilitation of the environment. Chem Eng J 415:129040
Lee B, Hadibarata T, Yuniarto A (2020) Phytoremediation mechanisms in air pollution control: a review. Water Air Soil Pollut 231(8):437
Li L, Li H, Zhang X, Wang L, Xu L, Wang X, Yu Y, Zhang Y, Cao G (2014) Pollution characteristics and health risk assessment of benzene homologues in ambient air in the northeastern urban area of Beijing, China. J Environ Sci 26(1):214–223
Liang H, Zhao S, Liu K, Su Y (2019) Roles of reactive oxygen species and antioxidant enzymes on formaldehyde removal from air by plants. J Environ Sci Health Part A Toxic-Hazard Subst Environ Eng 54(3-4):193–201
Linghu Y-w, Li B, Li S-f, Zhang Y, Liu L-c (2011) Monitoring, purification and response of three indoor ornamental plants on formaldehyde pollution. Acta Botan Boreali-Occiden Sin 31(4):776–782
Liu B, Ji J, Zhang B, Huang W, Huang H (2021a) Catalytic ozonation of VOCs at low temperature: a comprehensive review. J Hazard Mater 422(15):126847
Liu C, Huang X, Li J (2020) Outdoor benzene highly impacts indoor concentrations globally. Sci Total Environ 720(10):137640
Liu Q, Liu Y, Dong F, Sallach JB, Wu X, Liu X, Xu J, Zheng Y, Li Y (2021b) Uptake kinetics and accumulation of pesticides in wheat (Triticum aestivum L.): Impact of chemical and plant properties. Environ Pollut 275:116637
Lu S, Yang X, Li S, Chen B, Jiang Y, Wang D, Xu L (2018) Effects of plant leaf surface and different pollution levels on PM2.5 adsorption capacity. Urban For Urban Gree 34:64–70
Lu Y, Liu J, Lu B, Jiang A, Wan C (2010) Study on the removal of indoor VOCs using biotechnology. J Hazard Mater 182(1):204–209
Mosaddegh M, Jafarian A, Ghasemi A, Mosaddegh A (2014) Phytoremediation of benzene, toluene, ethylbenzene and xylene contaminated air by D. deremensis and O. microdasys plants. J Environ Health Sci Eng 12(1):1–7
Natarajan S, Mukhopadhyay K, Thangaswamy D, Natarajan A, Chakraborty D (2022) Characterisation of indoor volatile organic compounds and its association with respiratory symptoms among children living in solid fuel using households in Tamil Nadu, India. MAPAN-J Metrol Soc I 37(3):565–578
Oksanen E, Kontunen-Soppela S (2021) Plants have different strategies to defend against air pollutants. Curr Opin Environ Sci Health 19:100222
Parseh I, Teiri H, Hajizadeh Y, Ebrahimpour K (2018) Phytoremediation of benzene vapors from indoor air by Schefflera arboricola and Spathiphyllum wallisii plants. Atmos Pollut Res 9:1083–1087
Paull NJ, Irga PJ, Torpy FR (2019) Active botanical biofiltration of air pollutants using Australian native plants. Air Qual Atmos Hlth 12(12):1427–1439
Pérez DJ, Doucette WJ, Moore MT (2021) Contaminants of emerging concern (CECs) in Zea mays: uptake, translocation and distribution tissue patterns over the time and its relation with physicochemical properties and plant transpiration rate. Chemosphere 288:132480
Ptbsb A, Af A, Fgm A, Cf B, Lgv B, Sivs A (2020) Impact of indoor air pollution in nursery and primary schools on childhood asthma. Sci Total Environ 745:140982
Redondo-Bermúdez M, Gulenc IT, Cameron RW, Inkson BJ (2021) ‘Green barriers’ for air pollutant capture: leaf micromorphology as a mechanism to explain plants capacity to capture particulate matter. Environ Pollut 288:117809
Ren JC, Liu H, Zhang GH, Wang T, Xia ZL (2020) Dataset on the effect of benzene exposure on genetic damage, hematotoxicity, telomere length and polymorphisms in metabolic and DNA repair genes. Data Brief 31:105869
Sriprapat W, Thiravetyan P (2016) Efficacy of of ornamental plants for benzene removal from contaminated air and water: effect of plant associated bacteria. Int Biodeter Biodegr 113:262–268
Su Y, Liang Y (2013) The foliar uptake and downward translocation of trichloroethylene and 1, 2, 3-trichlorobenzene in air-plant-water systems. J Hazard Mater 252-253(15):300–305
Su Y, Liang Y (2015) Foliar uptake and translocation of formaldehyde with bracket plants (Chlorophytum comosum). J Hazard Mater 291(30):120–128
Teiri H, Pourzamani H, Hajizadeh Y (2018) Phytoremediation of VOCs from indoor air by ornamental potted plants: a pilot study using a palm species under the controlled environment. Chemosphere 197:375–381
Treesubsuntorn C, Suksabye P, Weangjun S, Pawana F, Thiravetyan P (2013) Benzene adsorption by plant leaf materials: effect of quantity and composition of wax. Water Air Soil Pollut 224(10):1–9
Wei Z, Le QV, Peng W, Yang Y, Yang H, Gu H, Lam SS, Wei Z, Le QV, Peng W, Yang Y, Yang H, Gu H, Lam SS, Sonne C (2020) A review on phytoremediation of contaminants in air, water and soil. J Hazard Mater 403:123658
Yang YX, Su YH, Zhao SY (2020) An efficient plant-microbe phytoremediation method to remove formaldehyde from air. Environ Chem Lett 18(2):527–527
Yue X, Ma NL, Sonne C, Guan R, Peng W (2020) Mitigation of indoor air pollution: a review of recent advances in adsorption materials and catalytic oxidation. J Hazard Mater 405:124138
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2022YFC3701403) and the Foundation of Xinjiang Educational Commission (XJEDU2021I07).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
Su Yuhong: conceptualization. Li Xiaojuan: methodology writing (review and editing, and original draft), and formal analysis. Hu Yuanfang: investigation. Li Depeng: visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare that no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Hu, Y., Li, D. et al. Transport and removal mechanism of benzene by Tradescantia zebrina Bosse and Epipremnum aureum (Linden ex André) G.S. Bunting in air-plant-solution system. Environ Sci Pollut Res 30, 58282–58294 (2023). https://doi.org/10.1007/s11356-023-26618-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26618-w