Abstract
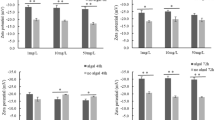
The extensive usage of iron oxide nanoparticles (FeO NPs) in commercial and biomedical applications raises the risk of releasing their remains into the aquatic ecosystems and this could possibly cause cytotoxic effects on aquatic organisms. Thus, the toxicity assessment of FeO NPs on cyanobacteria, which are primary producers at the bottom of food chain in aquatic ecosystems, is essential to gain information about the potential ecotoxicological threat on aquatic biota. The present study investigated the cytotoxic effects of FeO NPs on Nostoc ellipsosporum using different concentrations (0, 10, 25, 50 and 100 mg L−1) to track the time-dependent and dose-dependent effects and compared with its bulk equivalent. In addition, the impacts of FeO NPs and bulk counterpart on cyanobacterial cells were assessed under nitrogen as well as nitrogen-deficient conditions, because of ecological role of cyanobacteria in nitrogen fixation. The study revealed that the highest protein content was observed in the control in both types of BG-11 media compared to treatments of nano and bulk particles of Fe2O3. A 23% reduction in protein in nanoparticle treatment and a 14% reduction in bulk treatment at 100 mg L−1 was observed in BG-11 medium. At same concentration, in BG-110 media, this decline was even more intense with 54% reduction in nanoparticle and a 26% reduction in bulk. Catalytic activity of catalase and superoxide dismutase was found to be linearly correlated with the dose concentration for nano and bulk form in BG-11 as well as BG-110 media. The increased levels of lactate dehydrogenase act as biomarker of the cytotoxicity brought on by nanoparticles. Optical, scanning electron, and transmission electron microscopy all demonstrated the cell entrapment, nanoparticle deposition on the cell surface, cell wall collapse and membrane degradation. A cause for concern is that nanoform was found to be more hazardous than bulk form.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ANOVA:
-
Analysis of variance
- BG-11:
-
Blue green-11 media
- BG-110 :
-
Blue green-11 media without nitrogen
- EDTA:
-
Ethylenediamine tetraacetic acid
- Fe2O3 :
-
Iron oxide
- h:
-
Hours
- H2O2 :
-
Hydrogen peroxide
- M:
-
Molar
- MDA:
-
Malondialdehyde
- mg L− 1 :
-
Milligram per litre
- mL:
-
Milli litre
- mM:
-
Millimole
- NADH:
-
Nicotinamide adenine dinucleotide reduced
- NBT:
-
Nitro blue tetrazolium
- nm:
-
Nanometer
- PDI:
-
Polydispersity index
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- Tris-HCl:
-
Tris Hydrochloric acid
- μg:
-
Micro gram
- μmol:
-
Micro mole
References
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:1–13
Arunakumara K, Zhang X (2009) Effects of heavy metals (Pb2+ and Cd2+) on the ultrastructure, growth and pigment contents of the unicellular cyanobacterium Synechocystis sp. PCC 6803. Chin J Oceanol Limnol 27:383–388
Bhuvaneshwari M, Iswarya V, Archanaa S et al (2015) Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aquat Toxicol 162:29–38
Bian S-W, Mudunkotuwa IA, Rupasinghe T, Grassian VH (2011) Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 27:6059–6068
Bolade OP, Akinsiku AA, Oluwafemi OS et al (2021) Biogenic iron oxide nanoparticles and activated sodium persulphate for hydrocarbon remediation in contaminated soil. Environ Technol Innov 23:101719
Chu L, Hou X, Song X, Zhao X (2022) Toxicological effects of different ionic liquids on growth, photosynthetic pigments, oxidative stress, and ultrastructure of Nostoc punctiforme and the combined toxicity with heavy metals. Chemosphere 298:134273
Deng C, Tang Q, Yang Z et al (2022) Effects of iron oxide nanoparticles on phenotype and metabolite changes in hemp clones (Cannabis sativa L.). Front Environ Sci Eng 16:1–11
Du X, Zhou W, Zhang W et al (2021) Toxicities of three metal oxide nanoparticles to a marine microalga: Impacts on the motility and potential affecting mechanisms. Environ Pollut 290:118027
Ge F, Li M-M, Ye H, Zhao B-X (2012) Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J Hazard Mater 211:366–372
Gonzalez CM, Hernandez J, Peralta-Videa JR et al (2012) Sorption kinetic study of selenite and selenate onto a high and low pressure aged iron oxide nanomaterial. J Hazard Mater 211:138–145
Grover VA, Hu J, Engates KE, Shipley HJ (2012) Adsorption and desorption of bivalent metals to hematite nanoparticles. Environ Toxicol Chem 31:86–92
Guo H, Barnard AS (2013) Naturally occurring iron oxide nanoparticles: morphology, surface chemistry and environmental stability. J Mater Chem A Mater 1:27–42
Herbert D, Phipps PJ, Strange RE et al (1971) Chapter III chemical analysis of microbial cells. Methods Microbiol 5:209–344
Hochella MF, Aruguete D, Kim B, Madden AS (2012) Naturally occurring inorganic nanoparticles: General assessment and a global budget for one of Earth’s last unexplored major geochemical components. Nature’s nanostructures 1–31
Houimli SIM, Denden M, Mouhandes BD (2010) Effects of 24-epibrassinolide on growth, chlorophyll, electrolyte leakage and proline by pepper plants under NaCl-stress. Eurasian J Biosci 4
Huber DL (2005) Synthesis, properties, and applications of iron nanoparticles. Small 1:482–501. https://doi.org/10.1002/smll.200500006
Jin M, Wang H, Li Z et al (2019) Physiological responses of Chlorella pyrenoidosa to 1-hexyl-3-methyl chloride ionic liquids with different cations. Sci Total Environ 685:315–323
Kádár E, Lowe DM, Solé M et al (2010) Uptake and biological responses to nano-Fe versus soluble FeCl3 in excised mussel gills. Anal Bioanal Chem 396:657–666
Khoei AJ (2021) Evaluation of potential immunotoxic effects of iron oxide nanoparticles (IONPs) on antioxidant capacity, immune responses and tissue bioaccumulation in common carp (Cyprinus carpio). Comp Biochem Physiol c: Toxicol Pharmacol 244:109005
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mahfooz S, Jahan S, Shamim A et al (2018) Oxidative stress and response of antioxidant system in Nostoc muscorum exposed to different forms of Zinc. Turk J Biochem 43:352–361
Mahfooz S, Shamim A, Husain A, Farooqui A (2019) Physicochemical characterisation and ecotoxicological assessment of nano-silver using two cyanobacteria Nostoc muscorum and Plectonema boryanum. Int J Environ Sci Technol 16:4407–4418
Mahmoudi M, Laurent S, Shokrgozar MA, Hosseinkhani M (2011) Toxicity evaluations of superparamagnetic iron oxide nanoparticles: cell “vision” versus physicochemical properties of nanoparticles. ACS Nano 5:7263–7276. https://doi.org/10.1021/nn2021088
Metzler DM, Erdem A, Tseng YH, Huang CP (2012) Responses of algal cells to engineered nanoparticles measured as algal cell population, chlorophyll a, and lipid peroxidation: effect of particle size and type. J Nanotechnol 2012
Miri A, Najafzadeh H, Darroudi M et al (2021) Iron oxide nanoparticles: biosynthesis, magnetic behavior, cytotoxic effect. ChemistryOpen 10:327–333
Morales MI, Rico CM, Hernandez-Viezcas JA et al (2013) Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J Agric Food Chem 61:6224–6230
Nikookar K, Moradshahi A, Hosseini L (2005) Physiological responses of Dunaliella salina and Dunaliella tertiolecta to copper toxicity. Biomol Eng 22:141–146
Noori A, Parivar K, Modaresi M et al (2011) Effect of magnetic iron oxide nanoparticles on pregnancy and testicular development of mice. Afr J Biotechnol 10:1221–1227
Pakrashi S, Dalai S, Prathna TC et al (2013) Cytotoxicity of aluminium oxide nanoparticles towards fresh water algal isolate at low exposure concentrations. Aquat Toxicol 132:34–45
Pena RV, Machado RC, Caixeta MB et al (2022) Lauric acid bilayer-functionalized iron oxide nanoparticles disrupt early development of freshwater snail Biomphalaria glabrata (Say, 1818). Acta Trop 229:106362. https://doi.org/10.1016/j.actatropica.2022.106362
Rippka R, Deruelles J, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology (n y) 111:1–61
Roy R, Parashar A, Bhuvaneshwari M et al (2016) Differential effects of P25 TiO2 nanoparticles on freshwater green microalgae: Chlorella and Scenedesmus species. Aquat Toxicol 176:161–171
Salata OV (2004) Applications of nanoparticles in biology and medicine. J Nanobiotechnology 2:1–6
Saxena P, Saharan V, Baroliya PK et al (2021) Mechanism of nanotoxicity in Chlorella vulgaris exposed to zinc and iron oxide. Toxicol Rep 8:724–731
Saxena P, Sangela V, Harish (2020) Toxicity evaluation of iron oxide nanoparticles and accumulation by microalgae Coelastrella terrestris. Environ Sci Pollut Res 27:19650–19660. https://doi.org/10.1007/s11356-020-08441-9
Sayeed I, Parvez S, Pandey S et al (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26:2027–2038
Shi W, Guan X, Sun S et al (2020) Nanoparticles decrease the byssal attachment strength of the thick shell mussel Mytilus coruscus. Chemosphere 257:127200
Soenen SJH, de Cuyper M (2010) Assessing iron oxide nanoparticle toxicity in vitro: current status and future prospects. Nanomedicine 5:1261–1275
Soenen SJH, de Cuyper M (2009) Assessing cytotoxicity of (iron oxide-based) nanoparticles: an overview of different methods exemplified with cationic magnetoliposomes. Contrast Media Mol Imaging 4:207–219
Vakili-Ghartavol R, Mombeiny R, Salmaninejad A et al (2018) Tumor-associated macrophages and epithelial–mesenchymal transition in cancer: Nanotechnology comes into view. J Cell Physiol 233:9223–9236
Wang G, Hao Z, Huang Z et al (2010) Raman spectroscopic analysis of a desert cyanobacterium Nostoc sp. in response to UVB radiation. Astrobiology 10:783–788
Wu W, He Q, Jiang C (2008) Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett 3:397–415
Xiong D, Fang T, Yu L et al (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ 409:1444–1452
Yang H, Liu C, Yang D et al (2009) Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol 29:69–78
Yilancioglu K, Cokol M, Pastirmaci I et al (2014) Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS One 9:e91957
Zha S, Rong J, Guan X et al (2019) Immunotoxicity of four nanoparticles to a marine bivalve species, Tegillarca granosa. J Hazard Mater 377:237–248
Zha S, Tang Y, Shi W et al (2022) Impacts of four commonly used nanoparticles on the metabolism of a marine bivalve species. Tegillarca Granosa Chemosphere 296:134079
Zhang C, Liu T, Gao J et al (2010) Recent development and application of magnetic nanoparticles for cell labeling and imaging. Mini Rev Med Chem 10:194–203
Zhang H, Ji Z, Xia T et al (2012) Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 6:4349–4368
Zhang W, Gao J, Lu L et al (2021) Intracellular GSH/GST antioxidants system change as an earlier biomarker for toxicity evaluation of iron oxide nanoparticles. NanoImpact 23:100338
Acknowledgements
The authors thank AIIMS SAIF, New Delhi, for the HR-TEM and SEM- EDAX, MNIT Jaipur, Rajasthan, for the FE-SEM analysis, and UGC-DAE, Indore for the DLS and Zeta potential analysis. The authors also thank Prof. N. Laxmi, Prof. Sudhish Kumar and Dr. Prabhat K. Baroliya for their help with the analysis of XRD, AFM and FTIR, respectively.
Funding
The authors, Mukesh Kumar, Sunita Choudhary and Geetanjali Kumawat wish to acknowledge the support of the University Grants Commission (UGC), New Delhi, (Ref No. 191620001766 dated 20 July 2020), Council of Scientific and Industrial Research CSIR New Delhi, (Ref No. 09/172(0089)/2019-EMR-I) and UGC, New Delhi, (Ref No. 999/CSIR-UGC NET JUNE 2019), respectively. Financial support from the Department of Science and Technology, New Delhi, for laboratory infrastructure is acknowledged (SERB File Number: EEQ/2020/000011).
Author information
Authors and Affiliations
Contributions
Mukesh Kumar, experimentation, methodology, literature survey, writing – original draft; Kunal Seth, validation, writing – review and editing; Sunita Choudhary, validation, methodology; Geetanjali Kumawat, validation, methodology; Subhasha Nigam, validation, writing – review and editing; Garima Joshi, writing – review and editing; Vinod Saharan, writing – review and editing; Mukesh Meena, writing – review and editing; Amit Kumar Gupta, writing – review and editing; Harish, conceptualization, supervision, writing – review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
All authors have checked the manuscript and have agreed to the publication on ESPR.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, M., Seth, K., Choudhary, S. et al. Toxicity evaluation of iron oxide nanoparticles to freshwater cyanobacteria Nostoc ellipsosporum. Environ Sci Pollut Res 30, 55742–55755 (2023). https://doi.org/10.1007/s11356-023-26353-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26353-2