Abstract
Pesticide contamination of bee products is a widespread phenomenon. Due to its composition, bee bread is affected by both lipophilic and hydrophilic substances. As proof of concept of a monitoring campaign and to better understand the extent of contamination, we developed an analytical method based on a modified QuEChERS extraction, with subsequent separation by liquid chromatography and detection by mass spectrometry. This allowed for the quantitation of 51 agricultural- or beekeeping-associated pesticides in bee bread. The workflow was applied to 60 samples taken biweekly throughout spring to autumn 2022 from five colonies at a Swiss apiary in an agricultural area. In total, 30 pesticides were identified (> LOD), among which 26 pesticides were quantitated. The total number of pesticides detected per colony ranged from 11 to 19. The most prevalent substances (> LOQ) were two neonicotinoid insecticides, acetamiprid and thiacloprid (max. 16 μg/kg and 37 μg/kg, respectively); seven fungicides, azoxystrobin (max. 72 μg/kg), boscalid (max. 50 μg/kg), cyprodinil (max. 1965 μg/kg), difenoconazole (max. 73 μg/kg), mandipropamid (max. 33 μg/kg), pyraclostrobin (max. 8 μg/kg) and trifloxystrobin (max. 38 μg/kg); and two herbicides, prosulfocarb (max. 38 μg/kg) and terbuthylazine (max. 26 μg/kg). The study revealed strong variability in pesticide occurrence and concentrations among colonies sampled at the same site and date. The applied biweekly sampling of bee bread from March to August was shown to be reliable in capturing peak contaminations and revealing the onset of certain pesticides in bee bread. The study provides an adequate practical approach for pesticide monitoring campaigns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agriculture and floral ecosystems depend highly on honey bees (Apis mellifera) as pollinators. Honey bees commonly forage within a distance of 2 km, depending on the availability of food sources, and rarely fly more than 6 km (Visscher & Seeley, 1982). While foraging, honey bees are exposed to a plethora of environmental contaminants, including pesticides from agricultural activities (Murcia-Morales et al., 2022). When bees collect nectar, pollen, or water, they may also bring contaminants into their hives (Bogdanov, 2005). In this context, bee products collected by honey bees can serve for biomonitoring the foraging area with respect to environmental contaminants (van der Steen 2016).
Many studies have reported pesticide contamination of bee products (Beyer et al., 2018; Bokšová et al., 2021; Daniele et al., 2018; Orantes-Bermejo et al., 2015). Pesticides are frequently found in pollen (e.g., Friedle et al., 2021), beeswax (e.g., Marti et al., 2022; Wilmart et al., 2021), or honey (e.g., Souza Tette et al., 2016). Within the various hive compartments, lipophilic pesticides with a high logarithmic octanol-water partition coefficient tend to accumulate in beeswax. Over the past 30 years, Swiss beeswax has been tested for beekeeping-associated, lipophilic pesticides as part of the efforts to maintain good wax quality and, consequently, minimize the exposure of honey bees to pesticides in beeswax. Such monitoring has proven useful to prevent high levels of lipophilic pesticides in beeswax (Kast et al., 2021). However, a large variety of pesticides — both lipophilic and hydrophilic contaminants with a broad range of logarithmic octanol-water partition coefficients — may be primarily present in fresh or stored pollen (bee bread) (Murcia Morales et al., 2020). Hence, it is advantageous to include bee bread in a long-term monitoring program.
Pollen and bee bread serve as protein sources for larvae and newly emerged honey bees. Lack of pollen or low quality of pollen affects worker longevity (Di Pasquale et al., 2016). Furthermore, the development of the hypopharyngeal glands in young bees required later to produce larval jelly is highly dependent on protein intake (Di Pasquale et al., 2016), and nurse bees consume pollen and bee bread to provide a protein-rich jelly for larvae. Finally, well-fed larvae are a prerequisite for the rearing of healthy, long-lived winter bees, which are crucial for colony survival during winter months (Locke et al., 2014). Honey bees store part of the collected pollen as bee bread in the combs for later consumption. For storage, pollen is biochemically processed by enzymes derived from the saliva and gastric fluid of the honey bees (Giroud et al., 2013). Depending on brood presence and pollen availability in the environment, the amount of stored pollen may differ (Roessink & van der Steen, 2021). Field studies have shown that honey bees consume stored pollen (bee bread) preferably within a few days after collection (Carroll et al., 2017). Roessink and van der Steen (2021) demonstrated that 70% of bee bread is consumed within the first 5 days, whereas the remainder is consumed over 2 to 3 weeks. Therefore, little pollen older than 2 weeks is usually expected in bee bread at any given time during spring and summer. Hence, the analysis of bee bread should cover a collection period of up to two weeks. Sampling every second week most likely ensures a sufficient turnover of bee bread and so the bee bread predominantly includes pollen collected during the 2 weeks preceding the sampling. Based on this, bee bread was selected in this study as the matrix in order to cover a wide range of pesticides.
In an agricultural environment, honey bees may collect pollen and nectar from mass-flowering crops, like oilseed rape (OSR) and sunflowers, as well as pollen from maize (Requier et al., 2015). As OSR and sunflower are entomophilous taxa, they are dependent on pollination by honey bees. While these crops, along with maize, are attractive to honey bees, they are also often treated with pesticides, leading to the exposure of foraging bees to these substances. Consequently, these three taxa are the most researched plants in connection to neonicotinoid residues from agricultural practices impacting bees (Lundin et al., 2015).
Recently, efforts have been made to reduce the risk associated with the use of pesticides. In 2017, the Swiss government adopted an action plan with the aim to reduce the risk by 50% by the year 2027, using the years 2012–2015 as a reference period. Simultaneously, alternatives to chemical crop protection are being promoted and supported (Swiss Government, 2017, 2021). Hence, monitoring pesticides in bee bread might be useful to study the effectiveness of these political decisions.
The legislation in place regarding the allowance and use of pesticides in agricultural practices differs from one country to the other. Therefore, it is important to study contamination levels in each individual region of interest. Thus, our aim was to establish a method to monitor a variety of pesticides by analysing bee bread collected at an apiary in Switzerland. For this purpose, appropriate analytical methods were developed for the quantitation of a range of lipophilic as well as hydrophilic pesticides in bee bread. Firstly, sensitive, analytical methods for 51 pesticides were validated. Secondly, an apiary in an agricultural environment was chosen to evaluate ideal sampling time points, sampling frequency, and the minimal number of colonies needed to produce a robust temporal record of pesticide contamination in bee bread.
Material and methods
Chemicals and utilities
The following reference standards were obtained from LGC Standards GmbH (Wesel, Germany): acetamiprid (C10013000), aclonifen (C10042000), azoxystrobin (C10413000), bendiocarb (C10460000), boscalid (C10663000), bromopropylate (C10762000), chlorfenvinphos (C11290000), chlorpyrifos (C11600000), clothianidin (C11691700), clothianidin-D3 (C11691710), lambda-cyhalothrin (C11860000), zeta-cypermethrin (C11890500), cyproconazole (C11908000), cyprodinil (C11909000), deltamethrin (C12120000), dimethoate (C12700000), N-(2,4-dimethylphenyl)formamide (DMF) (C12737000), difenoconazole (C12609000), dimoxystrobin (C12775000), fenhexamid (C13476000), fenitrothion (C13480000), (E)-fenpyroximate (C13545000), fludioxonil (C13705000), flufenacet (C13711000), tau-fluvalinate (C13870000), flumethrin (C13719000), fluopyram (C13743000), hexythiazox (C14210000), imidacloprid (C14283700), indoxacarb (C14325500), iprovalicarb (C14371000), mandipropamid (C14745000), mepanipyrim (C14867000), metconazole (C14955000), methoxyfenozide (C15080500), permethrin (C15990000), piperonyl butoxide (C16240000), propoxur (C16500000), prosulfocarb (C16545000), desthio-prothioconazole (C16555500), pyraclostrobin (C16595000), spirodiclofen (C16972950), tebuconazole (C17178700), terbuthylazine (C17300000), thiamethoxam (C17453000), and trifloxystrobin (C17842000). Additionally, acrinathrin (46415), coumaphos (45403), flupyradifurone (37050), and thiacloprid (37905) were purchased from Sigma-Aldrich (Buchs, Switzerland), while fipronil (15900-2365-10AN10) was obtained from NEOCHEMA (Bodenheim, Germany).
Acetonitrile SupraSolv (1.00017) and 2-propanol (1.01040), LiChroSolv quality, were purchased from Merck (Darmstadt, Germany). Formic acid solution 50% for HPLC (09676) was acquired from Honeywell Fluka (Buchs, Switzerland) and ammonium formate (70221) from Supelco (Darmstadt, Germany). The calibrant mix G1969-85000 was obtained as tune solution from Agilent (Santa Clara, California, USA).
For the extraction process of pesticides in bee bread, the following substances were used: magnesium-sulfate (63136) and sodium hydrogencitrate sesquihydrate (359084) from Sigma-Aldrich (Buchs, Switzerland), sodium chloride (1.06404) and tri-sodium citrate dehydrate (1.06448.0500) from Merck (Darmstadt, Germany), and Bondesil PSA 40 μm (12213024), and Bondesil C18 40 μm (12213012) from Agilent Technologies (Santa Clara, California, USA).
A total of 1.5 mL Eppendorf Safe-Lock tubes (11.3119.06), 1.5 mL auto-sampler vials (190804.00), 2 mL single-use syringes (3.7410.02), and single-use polyamide filter (12.3663.01) were obtained from Huberlab (Aesch, Switzerland). Polystyrene Petri dishes (633180) and 50 mL polypropylene (PP) centrifugation tubes (227270) were purchased from Greiner Bio (St. Gallen, Switzerland).
Bee bread serving as a blank
The bee bread used as a blank extract or for spiking the pesticides to obtain recovery values was collected in 2015 and 2017 from several honey bee colonies owned by Agroscope, located in Liebefeld, Switzerland (46°55′46.9″N 7°25′26.8″E). In the urban environment, the overall contamination level of pesticides was low. Nevertheless, the bee bread contained azoxystrobin, trifloxystrobin, and difenoconazole at a concentration of approximately 3, 2, and 10 μg/kg, respectively.
Study site and honey bee colonies
The honey bee (Apis mellifera) colonies used in this study were located in an agricultural region in Northwestern Switzerland (46°58′57.6″N, 7°08′40.2″E). The site was chosen because it is surrounded by agricultural crops with cultivations of OSR, maize, sunflowers, and various vegetables where pesticide application is strongly expected. The occurrence and flowering periods of OSR, maize, and sunflower were noted during the sampling period, since they are attractive crops for honey bees. The colonies were treated against Varroa destructor infestation using organic acids the year before in August and December 2021. All colonies were overwintered in 12-frame Dadant-Blatt hives (the number of frames was reduced to 7 to 8 during winter), with combs up to about 3 years old. In 2022, treatment against V. destructor infestation was performed in four colonies, from 19th to 31st August, using a Nassenheider Pro (290 mL formic acid 60%, wick 2), while treatment was not necessary for one of the colonies. The colonies were fed with 5–7 L of syrup (60 % sugar) from 22nd July to 6th September 2022.
Bee bread sampling
Five colonies were selected based on a first inspection regarding the availability and quantity of stored bee bread in early spring. The first sampling served as a control, since it took place at the end of winter on 29th March 2022. Subsequently, samples were taken every second week from 15th April until 18th August 2022 (11 sampling dates) to cover a whole crop season. After formic acid treatment, a final sampling took place on 4th October 2022.
Whenever possible, two suitable combs containing fresh bee bread were chosen from each colony. From each comb, one rectangle (approximately 30 cm2) was cut out using a clean knife. The samples were stored at −20 °C until further use. To separate the bee bread from the comb, a tool designed by Gürle Aricilik (Nilüfer Bursa, Turkey) was used (Ürünler | gurlearicilik). It consists of a metal plunger with a spring that extracts the content of a cell. The bee bread from the two comb pieces of the same colony collected at each sampling date was combined and subsequently homogenized in a petri dish using a custom 3D-printed pestle (produced at Agroscope), resulting in sample weights of 5 to 15 g of bee bread. This procedure resulted in a total of 60 samples.
Extraction of pesticides
The extraction of pesticides followed a modified QuEChERS (quick, easy, cheap, efficient, rugged, safe) method, which was based on the procedure described by Niell et al. (2014). PSA and C18 sorbents were used for clean-up. Accordingly, 1 g of bee bread was weighed into a 50-mL PP centrifugation tube, 1 mL of MilliQ water was added, and then, the bee bread was suspended using a Vortex-Genie 2 mixer from Scientific Industries (New York, USA). After 15 min of resting, 4 mL of acetonitrile containing 50 μg/L clothianidin-D3 was added. The tubes were then vigorously shaken by hand three times for 30 s, with 15 min of rest in-between. Subsequently, a salt kit containing 0.6 magnesium sulphate, 0.2 g sodium chloride, 0.25 tri-sodium citrate dehydrate, and 0.12 g sodium hydrogencitrate sesquihydrate was added to salt out the aqueous phase. Next, the tubes were shaken for 10 min, using a UNIMAX 2010 sample shaker by Heidolph (Schwabach, Germany), to homogenize the samples. The tubes were then placed in the freezer at −20 °C for 1 h to freeze out the lipid and wax components. Subsequently, the samples were centrifuged at 4 °C and 10,000 g using a Sigma 4-16KS (Osterode am Harz, Germany). Afterwards, three 1 mL aliquots were pipetted into three separate 1.5 mL Safe-Lock tubes. One tube contained 50 mg Bondesil PSA, another contained 50 mg Bondesil C18, and the third one contained both 50 mg Bondesil PSA and 50 mg Bondesil C18. This separation was done since recoveries for cyprodinil and spinosad were found to be higher using only PSA, while using only C18 resulted in enhanced recoveries for fenhexamid and spirodiclofen. All other pesticides were extracted using both PSA and C18. The samples were then vortexed twice for 30 s. Subsequently, the samples were centrifuged at room temperature for 20 min using a Centrifuge 5804 from Eppendorf (Hamburg, Germany). The resulting supernatant was directly filtered into a 1.5-mL auto-sampler vial using a 2-mL single-use syringe coupled with a single-use polyamide filter of pore size 0.45 μm, before analysis with ultra-high-performance liquid chromatography (UHPLC) coupled to mass spectrometry (MS/MS).
UHPLC-MS/MS analysis
For the analysis of 51 pesticides, three methods (M1, M2, M3) with variable eluent gradients and ion source conditions were established. Liquid chromatography (LC) was performed using an Agilent 1290 Infinity II equipped with an autosampler and coupled with an Agilent 6495C tandem quadrupole mass spectrometer (MS). Chromatographic separation was performed at 40 °C on a C18 reverse phase column (Acquity UPLC HSS T3 Column, 100 Å, 1.8 μm, 2.1 mm × 100 mm) from Waters (Milford, Massachusetts, USA). The temperature of the autosampler was 10 °C, and the injection volume was 1 μL. The mobile phase A was 95% water + 5% acetonitrile + 0.01% formic acid + 5 mM ammonium formate, and the mobile phase B was 5% water + 95% acetonitrile + 0.01% formic acid + 5 mM ammonium formate. The LC gradient conditions for the three methods are shown in Table 1. The column flow was set to 0.5 mL/min. The solvents 2-propanol, acetonitrile, and 0.01 % formic acid in water were used as needle wash.
The detection and quantification of the pesticides were performed on an Agilent 6495C series tandem quadrupole MS system operating in electrospray ionization MS. The ion source conditions of the three methods are listed in Table 2. Methods 1 and 3 operated only in positive ionization mode, while method 2 included both positive and negative ionization modes (negative mode for fibronil). Quantitation and identification were based on selected ion transitions, thereby using one transition for quantitation and two additional transitions for identification. The ion transitions are listed in Table 3.
External matrix-matched calibration with nine concentration levels, ranging from 0.05 μg/L to 1000 μg/L, was used for quantitation. The sample concentrations were calculated based on the linear regression (1/x) of the calibration samples. All calculations were performed in Agilent MassHunter quantitative software Version B.08.00 (Basel, Switzerland).
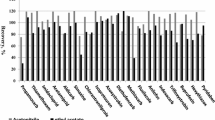
The deuterated substance clothianidin-D3 served as an internal standard to monitor the extraction performance. Furthermore, it served as a visual injection control for all pesticides, but no correctional factor was applied. The limit of detection (LOD) levels were experimentally determined for each pesticide by diluting spiked blank extracts (signal to noise ratio (s/n) at least 3:1). Recoveries were assessed for each pesticide at four to eight spiking levels, ranging from 0.2 to 10,000 μg/kg, with at least five repetitions per spiking level. Recoveries of the spiking levels were between 75 and 125% (except recovery of 69% for lowest spike level of tebuconazole at 5 μg/kg). The lowest spiking level of an individual pesticide, which showed a recovery of at least 75%, was set as its limit of quantification (LOQ) (except tebuconazole). The resulting LOD and LOQ values, including the validated concentration range, are given in Table 4. To survey the long-term consistency of instrument precision and accuracy, control samples with pesticide concentrations of 20 μg/kg or 1000 μg/were measured with each series.
Some pesticides were found in the blank bee bread, including azoxystrobin, trifloxystrobin, and difenoconazole at concentrations of approximately 3, 2, and 10 μg/kg, respectively. Therefore, the LOQs for these pesticides were set accordingly to 10, 5, and 10 μg/kg, respectively, while the LODs for these compounds were not determined (Table 4).
Results
An analytical procedure was validated, allowing for the quantitation of 51 pesticides in bee bread (Table 4). High sensitivity was achieved for 47 of the tested pesticides, with LOQs ranging between 0.2 and 10 μg/kg at recovery rates above 75% at the corresponding LOQ levels (except tebuconazole). The described analytical procedure was less sensitive for four of the tested pesticides with LOQs ranging from 20 to 100 μg/kg (bromopropylate, lambda-cyhalothrin, zeta-cypermethrin, deltamethrin).
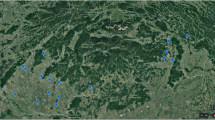
The cultivations of OSR, maize, and sunflower were mapped within a 2-km radius around the apiary during the crop season 2022 (Fig. 1). Here, maize fields made up the largest fraction among these three crop types, followed by OSR and sunflowers. The flowering periods were observed to be from 5th April to 15th May (OSR), 1st July to 1st September (maize), and 1st July to 25th July (sunflower).
The prevalence of pesticides present in bee bread is shown in Fig. 2. Out of the 51 compounds tested, 30 pesticides were identified (> LOD) at least in one of the samples, while 26 pesticides could be quantitated (> LOQ). The total number of pesticides detected per colony ranged from 11 to 19. Prosulfocarb, acetamiprid, pyraclostrobin, and desthio-prothioconazole were detected in at least 75% of all samples (> LOD). The prevalence of terbuthylazine, mandipropamid, cyprodinil, thiacloprid, and fluopyram was between 30 and 60%, while the prevalence of azoxystrobin, trifloxystrobin, boscalid, difenoconazole, permethrin, spinosad, flufenacet, indoxacarb, dimethoate, metconazole, fenpyroximate, and dimoxystrobin ranged from 10 to 30% (> LOD).
Two neonicotinoid insecticides (acetamiprid and thiacloprid), two herbicides (prosulfocarb and terbuthylazine), and seven fungicides (azoxystrobin, boscalid, cyprodinil, difenoconazole, mandipropamid, pyraclostrobin, and trifloxystrobin) were quantitated at least 10% of the samples (> LOQ) (Fig. 2). Other pesticides (fluopyram, permethrin, aclonifen, spinosad, tebuconazole, flufenacet, indoxacarb, dimethoate, fludioxonil) were quantitated in 3 to 8% of the samples. Yet others (desthio-prothioconazole, metconazole, lambda-cyhalothrin, iprovalicarb, piperonyl butoxide and fenhexamid) were quantitated (> LOQ) in just one of the samples. Additionally, the acaricide fenpyroximate, the insecticide chlorpyrifos, and the fungicides dimoxystrobin and cyproconazole were identified but could not be quantitated.
The temporal concentration profiles of the eleven most commonly quantified pesticides are shown in Fig. 3. Depending on the substance, they appeared differently at different points of time. Occurring in April, the earliest quantitated pesticides included the neonicotinoid insecticides acetamiprid and thiacloprid, the herbicide prosulfocarb, and the fungicide cyprodinil. Terbuthylazine was quantitated as early as mid-May 2022. The remaining six pesticides were quantifiable starting from June (mandipropamid, trifloxystrobin) or early July (azoxystrobin, boscalid, difenoconazole, pyraclostrobin) onwards. Five out of the eleven most commonly quantitated pesticides were still present in the last sampling at the beginning of October 2022 (acetamiprid, azoxystrobin, cyprodinil, difenoconazole, and trifloxystrobin).
The occurrence of thiacloprid in bee bread coincided with the blooming of OSR in spring. The appearance of terbuthylazine in bee bread from mid-May to early July occurred before the flowering stage of maize, while azoxystrobin was quantitated during the observed flowering periods of maize and sunflower in late summer (Fig. 3).
Even though the colonies were located only a few meters apart at the same apiary, the number of observations of each pesticide varied greatly between colonies (Fig. 3). One insecticide (acetamiprid), two herbicides (prosulfocarb, and terbuthylazine), and two fungicides (azoxystrobin, mandipropamid) were present in all colonies at least once throughout the sampling campaign. The fungicides cyprodinil and trifloxystrobin as well as the insecticide thiacloprid were quantifiable in four colonies, while the fungicides boscalid, difenoconazole, and pyraclostrobin were quantifiable in three out of five colonies (Figs. 3 and 4).
Temporal profile of cyprodinil in bee bread. The y-axis was enlarged to display low concentration levels. Four bars (green and grey on 29th April and black and green on 13th May) extend beyond the applied scale as indicated by the jagged top. The colonies are shown in different colours: Black (colony 1), green (colony 2), grey (colony 3), red (colony 4), and yellow (colony 5; not detected). The x-axis shows the dates corresponding to when a sample was taken
Furthermore, Fig. 3 shows large differences in pesticide levels. The highest residue levels up to a maximum concentration of 1965 μg/kg were measured for cyprodinil in one of the colonies. Azoxystrobin and difenoconazole were quantitated at a maximum of 72 μg/kg and 73 μg/kg, respectively. Lower maximum concentrations ranging from 25 to 50 μg/kg were measured for prosulfocarb, mandipropamid, thiacloprid, trifloxystrobin, terbuthylazine, and boscalid. In contrast, pyraclostrobin and acetamiprid were present at 8 to 16 μg/kg, respectively.
At any given date, the residue levels of pesticides in bee bread were found to differ strongly between the colonies, as the example of cyprodinil on 29th April 2022 shows (Figs. 3 and 4). While residue levels were present in Colony 2 at concentrations near 2000 μg/kg, concentrations in the other colonies’ bee bread were much lower (near 440 μg/kg in Colony 3, near 3 μg/kg in Colony 1), and it was not quantifiable in the remaining two colonies. Similar effects were observed for other pesticides, such as thiacloprid at the same date or terbuthylazine at the end of June 2022. These observations suggest different pollen compositions in bee bread from honey bee colonies located at the same site with the same pollen availability.
Discussion
A concept was established using honey bees for pesticide monitoring. The study revealed the presence of a large number of pesticides in the bee bread from a Swiss apiary in an agricultural area, suggesting that honey bee colonies are exposed to multiple pesticides. However, little is known about the health effects of such exposure. These circumstances justify using the current concept for monitoring a variety of apiaries to examine regional and transregional differences. In the future, the measured pesticide levels in bee bread as well as seasonal contamination profiles may act as the comparison baseline for future studies.
The presented workflow allowed for the analysis of 51 pesticides, both hydrophilic and lipophilic (log Kow between −0.1 and 7) with an appropriate method of QuEChERS extraction, UHPLC separation, and subsequent MS/MS quantification. The method was validated with LOQ values ranging from as low as 0.2 up to 10 μg/kg at recoveries ranging from 75 to 125% for 46 out of the 51 pesticides. The seasonal contamination profile of some pesticides correlated with the flowering periods of OSR (thiacloprid) early in the year and of maize (azoxystrobin) or sunflowers (azoxystrobin) in the late-season. This suggests that an adequate sampling should include flowering periods of crops important to honey bees. Furthermore, the variability of pesticide levels in bee bread between the tested colonies suggests that a study needs to include multiple colonies.
The current study was conducted at a single apiary. For an overview of the pesticide exposure of bees in Switzerland, multiple sites should be included in the future. Furthermore, our study gives little information on the crop types responsible for pesticides in bee bread. Ideally, a study would be conducted in a controlled environment where the date of spraying and the crops treated are known. This would allow conclusions to be drawn about the origin of the pesticides in bee bread.
Sampling time and frequency
Based on the various temporal pesticide profiles in bee bread, it becomes apparent that the time point of sample collection is crucial to the outcome of the study. Some pesticides were quantifiable throughout most of the sampling campaign (e.g., acetamiprid, prosulfocarb). Additional pesticides were quantifiable in early spring (e.g., thiacloprid) coinciding with the flowering period of OSR or early summer (e.g., terbuthylazine) during the seedling stage of maize, while yet others only appear later in the season (e.g., azoxystrobin) coinciding with the flowering periods of maize or sunflower. Thus, if the samples are not taken according to the flowering periods of crops attractive to honey bees, major contamination levels of pesticides in bee bread might be missed. Therefore, to produce contamination profiles across a full crop season, we suggest sampling ideally at least every 2 weeks. If only a smaller number of samples is achievable, we strongly suggest sampling during the flowering periods of major crops attractive to honey bees at the respective study site. However, restricting sampling to full bloom periods of crops attractive to honey bees may underestimate the contamination level, since herbicides or fungicides may be sprayed outside these periods. Additionally, pollen of wild plants might be contaminated by spray drift, thus increasing residual levels of pesticides in bee bread.
Number of colonies
Since the sampled colonies were located in close proximity, the pollen availability throughout the crop season was the same for all colonies. As described before, pollen composition can vary strongly between colonies at the same study site (Keller et al., 2015; Roncoroni et al., 2021). In the current study, variable pesticide levels might suggest discrepancies in foraging behaviour between colonies of the same apiary. Accordingly, differences in pesticide levels in the bee bread might indicate that forager bees collected dissimilar pollen types with variable contamination levels. For example, thiacloprid was not quantifiable in the bee bread of Colony 4 throughout the whole season, while bee bread from the other colonies showed distinctive contamination levels nearing maximal levels of 40 μg/kg. Consequently, forager bees as well as younger bees of Colony 4 were less exposed to this pesticide compared to the other colonies.
Evidently, our work showed that it is crucial to include a sufficiently large number of colonies per apiary to capture the in-between variabilities, especially for comparison of the contamination levels in transregional studies. We suggest pooling samples from several colonies to decrease the workload and costs, especially if the focus is mainly to determine the prevalence of contaminants in bee bread. However, the pesticide profiles will be smoothed and therefore maximum residue levels might be underestimated.
Flowering periods of relevant crops and occurrence of pesticides in beebread
The temporal occurrence of thiacloprid in bee bread, a neonicotinoid insecticide formerly designed as spray application in OSR (FSVO 2022), coincided with the observed flowering period of OSR from 5th April to 15th May. Thereby, the first occurrence of thiacloprid in bee bread was detected in mid-April, suggesting an application on OSR. Residues of thiacloprid were still present in the two subsequent samples after the end of the observed flowering period of OSR in the bee bread collected from one of the colonies (Colony 5). Hence, we might have missed some of the prolonged flowering OSR plants or thiacloprid may have remained on the pollen for a few more days. It is reported that the dissipation rate RL50 of thiacloprid (i.e., the rate at which it disappears due to processes such as volatilization or hydrolysis) on the plant matrix may be up to 11 days, as found in the pesticide properties database (Lewis & Tzilivakis, 2017; Lewis et al., 2016).
The use of thiacloprid was prohibited by the end of 2021 in the EU and Switzerland as part of phasing out harmful neonicotinoids (Eur-Lex, 2020; Fed-Lex, 2021). The relatively short dissipation rate of 11 days indicates that the residues are unlikely to originate from applications in 2021. Moreover, the first sampling in March did not contain thiacloprid residues, suggesting that the bee bread collected on April 15th consisted of newly collected pollen. Hence, residues from April samples were unlikely from unconsumed bee bread from the previous year. As a consequence, our findings suggest an improper application of thiacloprid in 2022 despite its ban. Subsequent monitoring in the following year would reveal if products containing thiacloprid are still actively applied.
Additionally, for the fungicide azoxystrobin, the findings correspond with the observed flowering period of maize from the beginning of July to August. According to the Swiss Pesticide Database, products containing azoxystrobin are approved for maize cultivations among other crops (FSVO 2022). For maize, azoxystrobin is included in products used as seed dressing with systemic properties (Bartlett et al., 2002). Furthermore, the pesticide is authorized as a spray application for sunflowers, when inflorescence buds between the young leaves are barely visible (stage BBCH 51). Thus, there is no applications of azoxystrobin expected during the blooming cultivation of maize and sunflower due to crop height. We, therefore, hypothesize that during the flowering of maize and sunflower, these pesticides are applied in cultures other than maize or sunflowers and are transported to the flower head of maize or sunflower due to wind drift from neighbouring crops. Additionally, flower heads and pollen of maize might contain azoxystrobin due to systemic transport in the plant (Bartlett et al., 2002).
Terbuthylazine is authorized for use as a herbicide for maize plants with permitted application up to leaf stage BBCH 10 to 16 (FSVO 2022). Terbuthylazine residues were quantifiable in mid-May and June, and thus, the occurrence of the residues correlates with an application during these leaf stages before the flowering of maize. Hence, maize pollen was not yet available. Therefore, we hypothesize that pollen containing terbuthylazine collected by bees during June originates from other cultures or wild plants that were exposed to drift during the spray treatment of the maize seedlings. Previous field studies and models produced by Simon-Delso et al. (2017) have shown that bees are additionally exposed to pesticides applied in crops that are less attractive food sources. They suggest spray drift and/or remobilization of persistent substances during crop rotation.
Comparison between our, EU, and non-EU studies
Comparison of our current findings from a single apiary with studies performed across Europe and overseas is challenging due to the varying study designs, especially for comparison of the maximal residue levels. Some studies were based on a single sampling time point (Orantes-Bermejo et al., 2015), while others sampled over a longer period but not in detail over a whole crop season (Tong et al., 2018). Additionally, sample numbers, numbers of apiaries, and spatial distribution of the apiaries were very variable. Furthermore, differences in analytical technique may result in different detection and quantification limits, thus affecting the number of pesticides detected.
In Germany, pesticide contamination of bee bread is regularly monitored as part of a national bee monitoring campaign (Deutsches Bienenmonitoring, 2021a, 2021b). In 2020, the study included 128 bee bread samples collected from 118 apiaries during spring (40), summer (83), and fall (5) (Deutsches Bienenmonitoring, 2021b). In 2019, 129 samples of bee bread from 110 apiaries were taken in spring and summer (Deutsches Bienenmonitoring, 2021a). In 2020, 83 pesticides were detected out of 457 analysed pesticides (Deutsches Bienenmonitoring, 2021b). In 2019, the detection rate was 90 out of 455 screened pesticides (Deutsches Bienenmonitoring, 2021a), which included all of the 11 most prevalent pesticides detected in our study. The distribution of the active substance classes was similar to that in our study. The most prevalent were fungicides, especially azoxystrobin and boscalid, followed by herbicides (e.g., prosulfocarb and terbuthylazine) and insecticides, especially thiacloprid. In Germany in 2019, maximum concentrations of the fungicides azoxystrobin, boscalid, prosulfocarb, and trifloxystrobin (max. 482, 339, 106 and 136 μg/kg) as well as the insecticide acetamiprid (max. 51 μg/kg) were found to exceed those of the studied Swiss apiary. On the other hand, the maximum level of the fungicide cyprodinil was 6-fold higher in our study (376 vs. 1965 μg/kg) as compared to Germany in the year 2019 (Deutsches Bienenmonitoring, 2021a). However, the samples of three colonies were pooled, and thus, residue levels of cyprodinil may have been higher in individual samples. Additionally, cyprodinil in bee bread at levels below that in the German study from 2019 have been reported from Italy (8–13 μg/kg) (Porrini et al., 2016) and Poland (< 10 μg/kg) (Kiljanek et al., 2021).
Furthermore, the fungicides azoxystrobin, difenoconazole, pyraclostrobin, and trifloxystrobin have been detected in bee bread from Luxembourg (Beyer et al., 2018), with azoxystrobin, trifloxystrobin, and thiacloprid exceeding maximum concentrations reported in our study. Azoxystrobin (Murcia Morales et al., 2020) and boscalid (Lozano et al., 2019) were reported in Spanish studies at low levels.
The insecticides thiacloprid and acetamiprid have also been found in bee bread samples from France (Daniele et al., 2018) and the Czech Republic (Bokšová et al., 2021) at levels above the maximum concentrations determined in our study. Furthermore, thiacloprid was detected in bee bread from Luxembourg (Beyer et al., 2018).
Overseas, eight of the twelve most prevalent pesticides in our study have been detected in a monitoring campaign performed in the USA by Traynor et al. (2021). They revealed high residue levels of cyprodinil (up to 5800 μg/kg), boscalid (up to 3070 μg/kg), azoxystrobin (up to 1870 μg/kg), and pyraclostrobin (up to 1070 μg/kg), while other pesticides were present at lower concentrations.
Toxicity to bees
A risk assessment based on the comparison of daily consumption of maximal contaminated bee bread compared to the respective oral acute lethal dose 50 (LD50) was performed to obtain an initial assessment of the residue levels in the analysed bee bread. Thereby, a maximum daily pollen uptake of 12 mg per adult nurse bee (Appendix J, Table J1, European Food Safety Authority, 2013) was used. Accordingly, the worst-case scenario of pesticide consumption for an adult nurse bee was determined by multiplying the measured maximum pesticide concentration in bee bread with the maximum daily pollen consumption (Table 5). The respective LD50 was divided by the maximal daily uptake per bee to yield the toxicity exposure ratio (TER). TER was calculated for all pesticides, except desthio-prothioconazole (transformation product of prothioconazole) and piperonyl butoxide, for which no oral acute toxicity data was available.
The lowest TER was determined for the insecticides spinosad and indoxocarb (TER 313 and 752) as well as for the pyrethroid permethrin (TER 515). While these risk quotients are well above the suggested threshold of 10, sublethal effects may not be disregarded. Recently, exposure to the biopesticide spinosad at environmentally realistic levels has been found to induce alterations in honey bee genes related to energy production (Christen et al., 2019). Similarly, exposure to indoxacarb has been determined to be toxic to honey bees at the recommended application dosage used in the field (Pashte & Patil Shivshankar, 2018).
Regarding the eleven most prevalent pesticides, the determined TER values show that the oral acute LD50 values exceed the calculated daily maximum pesticide uptake per bee by an order of magnitude 4 or higher. The summed consumption over the span of 10 days (assuming a nurse bee consuming only maximum contaminated bee bread) only lowers the toxicity exposure by one order of magnitude. Thus, the TER of the isolated concentrations determined in the bee bread is well above the respective oral acute LD50, even if such bee bread was consumed over a longer period of time. However, pesticide concentrations may not be evenly distributed between cells. Therefore, individual cells might contain higher pesticide concentrations. Furthermore, little is known about the additive and synergist toxicity of several pesticides, although these effects are suspected to play a greater role in honey bee decline than individual substances (Mullin et al., 2010).
Conclusion
This study serves as a proof of concept for environmental monitoring. It shows that honey bee colonies in an agricultural environment can be exposed to multiple pesticides. Our work conclusively reveals the advantage of a systematic sampling approach that resulted in a high temporal resolution record from one single apiary across a full crop season. The proposed biweekly sampling and the investigation of individual samples from several colonies has proven necessary to yield meaningful results for the studies site. Based on this systematic workflow, further steps may include sampling at multiple locations within Switzerland over several years, which will enable a comparison between the studied regions and the course of the years. This will allow an overview of pesticide exposure levels of honey bees in Switzerland and can be useful for monitoring the risk reduction measures based on policymaker decisions.
Data availability
The data generated during this study is included in this article and is also available from the corresponding author (christina.kast@agroscope.admin.ch). Samples are not available.
References
Bartlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B (2002) The strobilurin fungicides. Pest Manag Sci 58(7):649–662. https://doi.org/10.1002/ps.520
Beyer M, Lenouvel A, Guignard C, Eickermann M, Clermont A, Kraus F, Hoffmann L (2018) Pesticide residue profiles in bee bread and pollen samples and the survival of honey bee colonies-a case study from Luxembourg. Environ Sci Pollut Res Int 25(32):32163–32177. https://doi.org/10.1007/s11356-018-3187-4
Deutsches Bienenmonitoring (2021a) Abschlussbericht Zeitraum 2017-2019. Hohenheim University, Germany. Retrieved 18.02.2023 from https://bienenmonitoring.uni-hohenheim.de
Deutsches Bienenmonitoring (2021b) Zwischenbericht Zeitraum 2020. Hohenheim University, Germany. Retrieved 18.02.2023 from https://bienenmonitoring.uni-hohenheim.de
Bogdanov S (2005) Contaminants of bee products. Apidologie 37(1):1–18. https://doi.org/10.1051/apido:2005043
Bokšová A, Kazda J, Stejskalová M, Šubrt T, Uttl L, Mráz P, Bartoška J (2021) Findings of herbicide and fungicide residues in bee bread. Plant Soil Environ 67(6):343–352. https://doi.org/10.17221/135/2021-pse
Carroll MJ, Brown N, Goodall C, Downs AM, Sheenan TH, Anderson KE (2017) Honey bees preferentially consume freshly-stored pollen. PloS One 12(4):e0175933. https://doi.org/10.1371/journal.pone.0175933
Christen V, Krebs J, Bunter I, Fent K (2019) Biopesticide spinosad induces transcriptional alterations in genes associated with energy production in honey bees (Apis mellifera) at sublethal concentrations. J Hazard Mater 378:120736. https://doi.org/10.1016/j.jhazmat.2019.06.013
Daniele G, Giroud B, Jabot C, Vulliet E (2018) Exposure assessment of honey bees through study of hive matrices: analysis of selected pesticide residues in honey bees, beebread, and beeswax from French beehives by LC-MS/MS. Environ Sci Pollut Res Int 25(7):6145–6153. https://doi.org/10.1007/s11356-017-9227-7
Di Pasquale G, Alaux C, Le Conte Y, Odoux JF, Pioz M, Vaissiere BE, Belzunces LP, Decourtye A (2016) Variations in the availability of pollen resources affect honey bee health. PloS One 11(9):e0162818. https://doi.org/10.1371/journal.pone.0162818
Environmental Protection Agency (1997) Spinosad. Pesticide Fact Sheet
Eur-Lex (2020) Document 32020R0023. Retrieved 18.02.2023 from http://data.europa.eu/eli/reg_impl/2020/23/oj
European Food Safety Authority (2013) Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J 11(7). https://doi.org/10.2903/j.efsa.2013.3295
European Food Safety Authority (2018) Peer review of the pesticide risk assessment of the active substance spinosad. EFSA J 16(5). https://doi.org/10.2903/j.efsa.2018.5252
Fed-Lex. (2021). Verordnung über das Inverkehrbringen von Pflanzenschutzmitteln. AS 2021 321 Retreived 18.02.2023 from https://fedlex.data.admin.ch/eli/oc/2021/321
Friedle C, Wallner K, Rosenkranz P, Martens D, Vetter W (2021) Pesticide residues in daily bee pollen samples (April-July) from an intensive agricultural region in Southern Germany. Environ Sci Pollut Res Int 28(18):22789–22803. https://doi.org/10.1007/s11356-020-12318-2
FSVO (2022) Federal Food Safety and Veterinary Office, Switzerland, Pflanzenschutzmittelverzeichnis Retrieved 14.10.2022 from https://www.psm.admin.ch/de/produkte
Giroud B, Vauchez A, Vulliet E, Wiest L, Bulete A (2013) Trace level determination of pyrethroid and neonicotinoid insecticides in beebread using acetonitrile-based extraction followed by analysis with ultra-high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1316:53–61. https://doi.org/10.1016/j.chroma.2013.09.088
Kast C, Kilchenmann V, Charriere JD (2021) Long-term monitoring of lipophilic acaricide residues in commercial Swiss beeswax. Pest Manag Sci 77(9):4026–4033. https://doi.org/10.1002/ps.6427
Keller I, Fluri P, Imdorf A (2015) Pollen nutrition and colony development in honey bees: part 1. Bee World 86(1):3–10. https://doi.org/10.1080/0005772x.2005.11099641
Kiljanek T, Niewiadowska A, Malysiak M, Posyniak A (2021) Miniaturized multiresidue method for determination of 267 pesticides, their metabolites and polychlorinated biphenyls in low mass beebread samples by liquid and gas chromatography coupled with tandem mass spectrometry. Talanta 235:122721. https://doi.org/10.1016/j.talanta.2021.122721
Lewis K, Tzilivakis J (2017) Development of a data set of pesticide dissipation rates in/on various plant matrices for the Pesticide Properties Database (PPDB). Data 2(3). https://doi.org/10.3390/data2030028
Lewis KA, Tzilivakis J, Warner DJ, Green A (2016) An international database for pesticide risk assessments and management. Hum Ecol Risk Assess Int J 22(4):1050–1064. https://doi.org/10.1080/10807039.2015.1133242
Locke B, Forsgren E, de Miranda JR (2014) Increased tolerance and resistance to virus infections: a possible factor in the survival of Varroa destructor-resistant honey bees (Apis mellifera). PloS One 9(6):e99998. https://doi.org/10.1371/journal.pone.0099998
Lozano A, Hernando MD, Ucles S, Hakme E, Fernandez-Alba AR (2019) Identification and measurement of veterinary drug residues in beehive products. Food Chem 274:61–70. https://doi.org/10.1016/j.foodchem.2018.08.055
Lundin O, Rundlof M, Smith HG, Fries I, Bommarco R (2015) Neonicotinoid insecticides and their impacts on bees: a systematic review of research approaches and identification of knowledge gaps. PloS One 10(8):e0136928. https://doi.org/10.1371/journal.pone.0136928
Marti JNG, Kilchenmann V, Kast C (2022) Evaluation of pesticide residues in commercial Swiss beeswax collected in 2019 using ultra-high performance liquid chromatographic analysis. Environ Sci Pollut Res Int 29(21):32054–32064. https://doi.org/10.1007/s11356-021-18363-9
Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, Vanengelsdorp D, Pettis JS (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PloS One 5(3):e9754. https://doi.org/10.1371/journal.pone.0009754
Murcia Morales M, Gomez Ramos MJ, Parrilla Vazquez P, Diaz Galiano FJ, Garcia Valverde M, Gamiz Lopez V, Manuel Flores J, Fernandez-Alba AR (2020) Distribution of chemical residues in the beehive compartments and their transfer to the honey bee brood. Sci Total Environ 710:136288. https://doi.org/10.1016/j.scitotenv.2019.136288
Murcia-Morales M, Heinzen H, Parrilla-Vázquez P, Gómez-Ramos M, Fernández-Alba AR (2022) Presence and distribution of pesticides in apicultural products: a critical appraisal. TrAC Trends Anal Chem 146. https://doi.org/10.1016/j.trac.2021.116506
Niell S, Cesio V, Hepperle J, Doerk D, Kirsch L, Kolberg D, Scherbaum E, Anastassiades M, Heinzen H (2014) QuEChERS-based method for the multiresidue analysis of pesticides in beeswax by LC-MS/MS and GCxGC-TOF. J Agric Food Chem 62(17):3675–3683. https://doi.org/10.1021/jf405771t
Orantes-Bermejo FJ, Pajuelo AG, Megías MM, Fernández-Píñar CT (2015) Pesticide residues in beeswax and beebread samples collected from honey bee colonies (Apis mellifera L.) in Spain. Possible implications for bee losses. J Apic Res 49(3):243–250. https://doi.org/10.3896/ibra.1.49.3.03
Pashte V, Patil Shivshankar C (2018) Toxicity and poisoning symptoms of selected insecticides to honey bees (Apis mellifera L.). Arch Biol Sci 70(1):5–12. https://doi.org/10.2298/abs170131020p
Porrini C, Mutinelli F, Bortolotti L, Granato A, Laurenson L, Roberts K, Gallina A, Silvester N, Medrzycki P, Renzi T, Sgolastra F, Lodesani M (2016) The status of honey bee health in Italy: results from the nationwide bee monitoring network. PloS One 11(5):e0155411. https://doi.org/10.1371/journal.pone.0155411
PubChem. (2022a). Dimoxystrobin. Retrieved 29.11.2022 from https://pubchem.ncbi.nlm.nih.gov/compound/dimoxystrobin#section=Computed-Properties&fullscreen=true
PubChem. (2022b). Fipronil. Retrieved 29.11.2022 from https://pubchem.ncbi.nlm.nih.gov/compound/3352#section=LogP
Requier F, Odoux JF, Tamic T, Moreau N, Henry M, Decourtye A, Bretagnolle V (2015) Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds. Ecol Appl 25(4):881–890. https://doi.org/10.1890/14-1011.1
Roessink I, van der Steen JJM (2021) Beebread consumption by honey bees is fast: results of a six-week field study. J Apic Res 60(5):659–664. https://doi.org/10.1080/00218839.2021.1915612
Roncoroni F, Kilchenmann V, Bieri K, Ritter R, Kast C (2021) Pollensammelverhalten von Völkern am gleichen Standort. Schweizerische Bienen Zeitung 2:16–19
Simon-Delso N, San Martin G, Bruneau E, Delcourt C, Hautier L (2017) The challenges of predicting pesticide exposure of honey bees at landscape level. Sci Rep 7(1):3801. https://doi.org/10.1038/s41598-017-03467-5
Souza Tette PA, Rocha Guidi L, de Abreu Gloria MB, Fernandes C (2016) Pesticides in honey: a review on chromatographic analytical methods. Talanta 149:124–141. https://doi.org/10.1016/j.talanta.2015.11.045
Swiss Government (2017) Aktionsplan zur Risikoreduktion und nachhaltigen Anwendung von Pflanzenschutzmitteln. Retrieved 29.11.2022 from https://www.blw.admin.ch/blw/de/home/nachhaltige-produktion/pflanzenschutz/aktionsplan.html
Swiss Government (2021) Bundesgesetz über die Verminderung der Risiken durch den Einsatz von Pestiziden. Retrieved 29.11.2022 from https://www.bk.admin.ch/ch/d/pore/rf/cr/2021/20210841.html
Tong Z, Duan J, Wu Y, Liu Q, He Q, Shi Y, Yu L, Cao H (2018) A survey of multiple pesticide residues in pollen and beebread collected in China. Sci Total Environ 640-641:1578–1586. https://doi.org/10.1016/j.scitotenv.2018.04.424
Traynor KS, Tosi S, Rennich K, Steinhauer N, Forsgren E, Rose R, Kunkel G, Madella S, Lopez D, Eversole H, Fahey R, Pettis J, Evans JD, Dennis, v. (2021) Pesticides in honey bee colonies: establishing a baseline for real world exposure over seven years in the USA. Environ Pollut 279:116566. https://doi.org/10.1016/j.envpol.2021.116566
van der Steen JJM (2016) The colony of the honey bee (Apis Mellifera L) as a bio-sampler for pllutants and plant pathogens. Wageningen University. https://doi.org/10.18174/375348
Visscher PK, Seeley TD (1982) Foraging strategy of honey bee colonies in a temperate deciduous forest. Ecology 63(6). https://doi.org/10.2307/1940121
Wilmart O, Legreve A, Scippo ML, Reybroeck W, Urbain B, de Graaf DC, Spanoghe P, Delahaut P, Saegerman C (2021) Honey bee exposure scenarios to selected residues through contaminated beeswax. Sci Total Environ 772:145533. https://doi.org/10.1016/j.scitotenv.2021.145533
Acknowledgements
We thank Jean-Daniel Charrière for taking the time to discuss the project, providing suggestions, and conducting a critical review of the manuscript with respect to the use of plant protection products in Switzerland. We also thank Daniela Grossar for providing help with the interpretation of the results with respect to bee toxicity and Adrien von Virag for his sporadic help in sample collection at the apiary.
Funding
Open access funding provided by Agroscope Open access funding provided by Agroscope.
Author information
Authors and Affiliations
Contributions
CK, MF, and BD designed the experiments. CK supervised the project. MF and CK validated the analytical method. MF, ES, and BD collected the bee bread samples. BD was the beekeeper of the apiary and also noted the locations of the crop plantations and the corresponding flowering periods. ES performed the analysis of the bee bread samples. ES and CK interpreted the data. ES wrote the paper, and CK revised it. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schaad, E., Fracheboud, M., Droz, B. et al. Quantitation of pesticides in bee bread collected from honey bee colonies in an agricultural environment in Switzerland. Environ Sci Pollut Res 30, 56353–56367 (2023). https://doi.org/10.1007/s11356-023-26268-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26268-y