Abstract
Brachionus plicatilis is a cosmopolitan rotifer used as a model organism in several research areas and as live food in aquaculture. Being a species complex, responses to stressors vary even among strains of the same species and, thus, the responses of one species are not representative of the whole complex. This study aimed to address the effects of extreme salinity ranges, and different concentrations of hydrogen peroxide, copper, cadmium, and chloramphenicol, in two strains of B. koreanus (MRS10 and IBA3) from B. plicatilis species complex, by assessing effects on their survival and swimming capacity. Neonates (0–4 h old) were exposed to the stressors in 48 well-microplates, for 24 and 6 h, to evaluate lethal and behavioural effects, respectively. Tested conditions of chloramphenicol did not show any effects on rotifers. The behavioural endpoint showed to be particularly sensitive to assess the effects of high salinity, hydrogen peroxide, and copper sulfate, as swimming capacity impairment was observed for both strains in the lowest concentrations used in lethal tests. Overall, results showed that IBA3 was more tolerant to the majority of stressors, comparing to MRS10, which may be due to differences in physiological characteristics, highlighting the importance of performing multiclonal experiments. Also, swimming capacity inhibition proved to be a good alternative to the classical lethality tests, being sensitive to lower concentrations and with shorter exposure periods.
Similar content being viewed by others
Introduction
As members of marine and freshwater zooplankton communities, rotifers are a relevant group of organisms in aquatic ecosystems connecting phytoplankton and upper trophic levels, while also playing an important role in aquaculture industry as larvae food (Chen et al. 2014; Yúfera 2001). Among rotifers, the monogonont rotifer Brachionus plicatilis (Müller, 1786) is one of the best studied taxa and, due to its cosmopolitan distribution in marine habitats, one of the most used in aquaculture (Lubzens 1987).
Rotifers have been also particularly useful for research in different areas given their small size, high ingestion rate, high growth rate, ease of culture in small volumes, short generation time, reproduction mainly via parthenogenesis (genetic homogeneity), and sensitivity to various toxicants (Marcial et al. 2005; Papakostas et al. 2006; Garaventa et al. 2010). Due to these characteristics, in the last decades B. plicatilis has been widely used as a model organism in basic research, as well as a bio-indicator and model for ecotoxicology (extensively reviwed by Snell and Janssen 1995; Dahms et al. 2011; Kostopoulou et al. 2012; Rico-Martínez et al. 2013, 2016; Li et al. 2020).
Although several toxicity tests have been developed to assess endpoints such as behaviour, ingestion rates, enzymatic activity, genetic expression, and life table parameters, most of the available studies used 24 h or 48 h lethality tests as preferred endpoints (Rico-Martínez et al. 2016). However, as emphasised by Rebolledo et al. (2020), from a population point of view, chronic tests (e.g. life tables) give a more realistic outcome of the impacts of toxicants in rotifers. On the other hand, these tests require a considerable amount of time, and thus behaviour endpoints have been considered as a viable alternative for their fast assessment of effects (Dahms et al. 2011). A good behavioural response should be specific, sensitive to a wide range of stressors, ecologically relevant, and transversal to different species, which are requirements met by the swimming behaviour of aquatic organisms (Garaventa et al. 2010; Melvin and Wilson 2013). Swimming behaviour has high ecological relevance, since processes such as conspecific recognition, feeding, predator avoidance, and mating when reproducing sexually, are dependent on individual swimming capacity. Therefore, swimming activity assays can give information at a realistic level, since alterations in this endpoint may indirectly predict changes in growth and survival of a population exposed to a contaminant (Chen et al. 2014). Also, due to its sensitiveness to several contaminants at low concentrations, swimming activity has been proposed as a promising tool for toxicity tests in rotifers.
Concerning the susceptibility to toxic substances, large differences have been observed not only among members of the same genus, revealing a need to test several species to assess the effects of contaminants in rotifers (Dahms et al. 2011; Pérez-Legaspi and Rico-Martínez 2001), but also among clones or strains, highlighting the importance of multiclonal experiments (Campillo et al. 2011; Rico-Martínez et al. 2016). This is particularly relevant for B. plicatilis since, having once been considered a species with morphological variability, more recent taxonomic and genetic studies revealed it is in fact a species complex (Serra and Fontaneto 2017). Studies have been conducted to better understand the differences among biotypes, addressing parameters such as growth and reproduction (Gómez et al. 1997; Granada et al. 2022; Hagiwara et al. 1995), morphology (Ciros-Pérez et al. 2001; Hwang et al. 2013), lifespan (Gribble et al. 2014; Kaneko et al. 2011; Snare et al. 2013), response to toxicants (Han et al. 2023; Kang et al. 2019), and others.
Thus, the main objective of this work was to evaluate the sensitiveness of the swimming capacity endpoint to assess differences in the response between two strains of B. koreanus (from B. plicatilis species complex) to a range of different abiotic stressors, including extreme abiotic conditions (low and high salinity), two metals (copper and cadmium), and substances used in aquaculture for disinfection processes (hydrogen peroxide) and to obtain axenic cultures (chloramphenicol).
Material and methods
Rotifer cultures
Clone cultures of two strains, MRS10 and IBA, of monogonont rotifer B. koreanus were used in this study. Species identification was confirmed by analysis of 16S rRNA genome sequences (Granada et al. 2022).
Cultures were constituted only by parthenogenetic females, reducing the intraspecific variability in their response to stressors. Cultures were maintained at 25 psu (autoclaved artificial seawater, ASW, Instant Ocean Sea Salt with deionized water), room temperature of 25 ± 1 °C, under constant light intensity of 34 µmol m−2 s−1 (cool white tube lights). Cultures were fed daily with Tetraselmis sp. at a final concentration of 105 cells mL−1 (Granada et al. 2022).
Chemicals
Hydrogen peroxide (30%; H2O2; Merck KGaA, Darmstadt, Germany), copper sulfate (CuSO4.5H2O; 249.68 g mol−1; Alfa Aesar, Ward Hill, MA, USA; 99% purity), cadmium chloride (CdCl2; 183.32 g mol−1; Merck, Darmstadt, Germany; purity ≥ 99.99%), and chloramphenicol (C11H12Cl2N2O5; 323.13 g mol−1; Merck, Darmstadt, Germany; purity ≥ 98%) were used according to the instructions of the manufacturer. Highly concentrated stock solutions were prepared in ASW or Milli-Q water and diluted in ASW to prepare the test solutions with different concentrations. The same stock solution was used to test each strains’ responses to a given stressor. Ethanol was used as solvent to prepare the stock solution of chloramphenicol.
Exposure setup
The effects of salinity, hydrogen peroxide, copper sulfate, cadmium chloride, and chloramphenicol were assessed on the two strains of rotifers (MRS10 and IBA3), addressing survival and behaviour endpoints. Every assay was performed in 48-well microplates, using 0–4 h old neonates of clone cultures, and consisting of 500 µL of medium per well, where six treatments of a given stressor were tested with six replicates of five rotifers per treatment. Tests were conducted in a climatic room at 25 ± 1 °C, with no light, and rotifers were not fed during exposures.
Lethality tests
The acute toxic effects were evaluated following the procedures described in ISO 19820: 2016(E). Briefly, newly hatched neonates (0–4 h old) were exposed to every single stressor for 24 h. Neonates of both strains were challenged with low (0, 0.5, 1.0, 1.5, 2.0, 2.5 psu) and high (60, 65, 70, 75, 80, 85 psu) salinity conditions, hydrogen peroxide (0, 2.21, 2.33, 2.45, 2.58, 2.72 mg L−1), copper sulfate (0, 0.50, 0.78, 1.22, 1.92, 3.00 mg L−1), cadmium chloride (0, 30.00, 60.62, 122.47, 247.46, 500.00 mg L−1), and chloramphenicol (0, 1.00, 3.16, 10.00, 31.62, 100 mg L−1). For chloramphenicol, a solvent control was used consisting of ASW with 0.20% ethanol (v/v), corresponding to the maximum solvent concentration used in the chloramphenicol treatments (highest treatment — 100 mg L−1).
At the end of the experiment, the number of live rotifers was determined, and the concentration causing 50% lethality (LC50) was calculated for each stressor and respective strain. Rotifers were considered dead if not showing any movement during 10 s of observation. Tests were considered valid if the percentage of mortality in the negative control was not higher than 10% (ISO 19820: 2016).
Behaviour response
Behaviour effects were evaluated by analysing the swimming capacity of organisms after the exposure to the above-mentioned stressors.
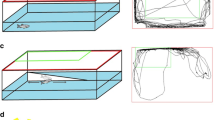
Newly hatched neonates were exposed to the same concentrations of the lethality tests for 6 h to assess the effect of stressors on the swimming speed, according to Liang et al. (2018). As movement impairment was observed in the lethality tests already in the lower concentration tested for high salinity conditions, hydrogen peroxide, and copper sulfate, a lower range was used to perform the behaviour tests (high salinity: 40, 45.95, 52.78, 60.63, 69.64, 80 psu; hydrogen peroxide: 0, 0.34, 0.54, 0.87, 1.38, 2.21 mg L−1; copper sulfate: 0, 0.10, 0.19, 0.36, 0.68, 1.30 mg L−1). At the end of the experiment, and before the observation, the volume of each well was reduced to 150 µL to avoid up and down movement in the water column. Each well was recorded for 1 min under a magnifying glass, using the camera AxioCam (Zeiss Microscopy) with 1.4 MP of resolution, connected to the ZEN software (Zeiss Microscopy). All movies were exported under AVI uncompressed format (.avi) and, for each rotifer, trajectories were manually analysed and mean speed calculated, using the image processing plugin MTrackJ, of ImageJ software (Schneider et al. 2012; Yúfera et al. 2005) (Fig. 1). These values were used to calculate the swimming inhibition rate normalized to the average values in control treatment, by the equation: Inhibition rate (%) = [(Vcontrol – Vtreatment)/Vcontrol] × 100 (Chen et al. 2014), where Vcontrol is the average values of swimming velocity in the control treatment of a stressor, and Vtreatment is the swimming velocity in the treatments where rotifers were exposed to that stressor. Values of inhibition rate were used to calculate the swimming capacity of rotifers, by the equation: Swimming capacity (%) = 100 – Inhibition rate, considering a swimming capacity under control conditions of 100%.
Example of trajectories obtained and analysed when rotifers were exposed to (a) control condition, (b) middle condition, and (c) maximum condition of a stressor. These trajectories were obtained from the exposure of MRS10 neonates to hydrogen peroxide (0 mg L−1, 0.87 mg L−1, and 2.21 mg L−1, respectively)
Tests were considered valid if the percentage of organisms with swimming capacity impairment in the negative control was not higher than 20%.
Statistical analysis
In order to evaluate acute and behaviour effects of each stressor on both strains, 24 h-LC50 (50% reduction in survival compared to control treatments) and 6 h-EC50 (50% reduction in swimming speed compared to control treatments) values were analysed. Firstly, LC50 and EC50 values, and respective 95% confidence intervals, were calculated based on log concentrations by fitting four-parameter logistic dose–response curves (Y = Bottom + (Top–Bottom)/(1 + 10(LogEC50−X)*HillSlope)), where “Y” is the response (% survival for LC50 estimation; % swimming capacity for EC50 estimation); “Bottom” the basal response (0%), “Top” the maximal response (100%), “X” the logarithm of concentration, and “HillSlope” the slope of the logistic curve. Secondly, to compare the concentrations of effect for each stressor between strains, a global fitting (extra sum of squares F-test) was performed. All these analyses were done using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA, USA).
Exceptionally, as in behaviour tests both strains showed a hormetic response to hydrogen peroxide, and MRS10 to cadmium chloride, the EC50 values in these cases were calculated based on the model: EC50 = (t*(1 + h*Conc))/(1 + ((0,5 + h*Conc)/0.5)*(Conc/x)b), where “t” is the control response, “h” the hormetic effects (estimated between 0.1 and 1), “Conc” the exposure concentration, and “b” a scale parameter (estimated between 1 and 4). These data were analysed using STATISTICA software (StatSoft, Inc., Tulsa, OK, USA).
To assess differences between average velocities in control treatments between strains, independent T-tests were performed using the IBM SPSS Statistics 26. Results are presented as mean ± SD. For all statistical tests, the significance level was set at P ≤ 0.05.
All graphics were made using GraphPad Prism version 6.00 for Windows.
Results
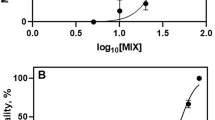
All lethality tests fulfilled the validity criteria as described by the standard guideline (ISO 19820: 2016). Survival results after exposure to low and high salinity, hydrogen peroxide, copper sulfate, cadmium chloride, and chloramphenicol can be seen in Fig. 2, for both strains. The 24 h-LC50 values, along with the global fitting comparisons between strains and respective statistical results are given in Table 1.
Concerning the exposure to low salinity conditions, IBA3 showed to be more tolerant with significantly lower LC50 (1.09 psu; F2,68 = 16.18, P < 0.0001) than MRS10 (1.35 psu). At high salinity conditions, MRS10 and IBA3 did not show statistically significant differences, with LC50 values of 70.13 psu and 70.47 psu, respectively. MRS10 were more tolerant to hydrogen peroxide, with a significantly higher LC50 (2.42 mg L−1; F2,68 = 12.87, P < 0.0001) in comparison with IBA3 (2.27 mg L−1). MRS10 showed to be more sensitive to copper sulfate, with a significantly lower LC50 (0.65 mg L−1; F2,68 = 10.26, P = 0.0001) when compared with IBA3 (0.81 mg L−1). MRS10 was more sensitive to cadmium chloride as well, with a significantly lower LC50 (130.8 mg L−1; F2,68 = 36.69, P < 0.0001) when compared with IBA3 (210.5 mg L−1). For chloramphenicol it was not possible to calculate LC50 values, as the concentrations used (0–100 mg L−1) did not have any effects on both strains survival. For this stressor, no statistically significant differences were found between control and solvent control for both lethality and behavioural tests (P < 0.05).
All behaviour tests fulfilled the established validity criteria. Swimming capacity results after exposure to low and high salinity, hydrogen peroxide, copper sulfate, cadmium chloride, and chloramphenicol can be seen in Fig. 3, for both strains. The 6 h-EC50 values, along with the global fitting comparisons between strains and respective statistical results are given in Table 2.
The average swimming velocities in control treatments for MRS10 and IBA3 were 138.00 ± 68.34 and 150.67 ± 57.47 µm sec−1, respectively, not showing statistically significant differences (P = 0.735). These velocities represent the normal swimming capacity of rotifers used in this study.
For low salinity, as verified for the acute lethality effects, IBA3 was more tolerant than MRS10 (F2,58 = 6.753, P = 0.0023), with an EC50 value of 1.068 psu in comparison with 1.363 psu for MRS10. Regarding high salinity, IBA3 was also more tolerant than MRS10 (F2,58 = 42.96, P < 0.0001), with an EC50 value of 60.98 psu, comparing with 49.55 psu for MRS10. For hydrogen peroxide, no statistically significant differences between strains were observed (F2,58 = 2.633, P = 0.0805), with MRS10 showing an EC50 value of 0.66 mg L−1 and IBA3 of 0.59 mg L−1. Contrary to the acute test, MRS10 showed to be more tolerant to copper sulfate, with a significantly higher EC50 (0.1341 mg L−1; F2,58 = 6.848, P = 0.0021) when compared with IBA3 (0.0877 mg L−1). The range of concentrations used in the cadmium exposures showed to affect the swimming capacity of rotifers, and the EC50 values were calculated as 272.14 mg L−1 and 230.10 mg L−1, for MRS10 and IBA3, respectively, being MRS10 more tolerant (F2,46 = 4.639, P = 0.0146). While both strains showed a logistic response to the overall stressors, a hormetic response was observed for hydrogen peroxide for both strains, and for cadmium chloride for MRS10 strain, under the tested concentrations. As for chloramphenicol, and similarly to the observed for the acute effects, no alterations on the swimming capacity of both strains were verified, considering the tested concentrations.
Discussion
In this study, the susceptibility of two strains of the B. plicatilis species complex, identified as B. koreanus, to extreme ranges of salinity, two metals (one essential and one non-essential), one peroxide compound, and one antibiotic, was compared by addressing lethal and behavioural endpoints.
Differences in sensitivity between strains — effects on survival
Overall, IBA3 showed higher tolerance than MRS10 to the tested stressors in terms of survival, except for hydrogen peroxide, to which it was more sensitive than MRS10, and for high salinity, to which there were no differences between strains.
In the aquatic environment, where organisms are immersed in a liquid and dependent of the medium characteristics, salinity is one of the most important abiotic factors, affecting biological processes from the organism to the ecosystem level (Lee et al. 2017). Seasonal and spatial fluctuations in salinity significantly affect rotifer abundance by changing its growth and fecundity, and regulates competition between species in natural habitats (Kim et al. 2020). In general, rotifer B. plicatilis is euryhaline, naturally occurring at salinities between 2 and 65 psu, and being able to tolerate a wider salinity range (1 to 97 psu) (Fielder et al. 2000; Han and Lee 2020; Lowe et al. 2005). However, it is important to notice that, being a species complex, it comprises species and strains with different optimal salinities that, along with other abiotic and biotic factors, mediate their coexistence (Montero-Pau et al. 2011). In the present study, newly hatched neonates were exposed to low and high salinities, with no observed mortality at 2.5 and 60 psu for both strains. Although the results for low salinity were expected, MRS10 and IBA3 appeared to be more tolerant to high salinities than other studies with B. plicatilis where total mortality of neonates was verified at 39 and 40 psu (Han and Lee 2020; Joshi 1988). The LC50 values (Table 1) obtained at high salinity for both strains (70.13 and 70.47 psu) were however more consistent with the 68.1 psu observed by Yoon and Park (2011) for B. plicatilis neonates exposed to brine discharges by desalination industries.
As aquatic organisms, rotifers are also often exposed to chemical products resultant from anthropogenic activities, industry, agriculture, and aquaculture (Jeong et al. 2019). Toxicity studies with several rotifer species, mainly from freshwater environments, have been conducted with a wide range of inorganic (metals and metalloids) and organic compounds (e.g. pesticides, detergents, solvents, colorants), and pharmaceuticals (Rico-Martínez et al. 2016). In this study, the effects of the metals copper sulfate (recommended as reference substance in ISO 19820: 2016) and cadmium chloride were assessed for comparison purposes between strains. Copper is considered an essential metal due to its biological function (Jeong et al. 2019) since, at low concentrations, is necessary for several enzymatic and molecular functions; however, at higher concentrations can be cytotoxic and, in rotifers, has been shown to cause high mortality and abnormal behaviour (Cooper et al. 2014; Han et al. 2013). The 24 h-LC50 values observed for MRS10 and IBA3 (0.65 mg L−1 and 0.81 mg L−1, respectively; Table 1) were within the interval observed in other studies with B. koreanus (0.13–1.2 mg L−1) (Han et al. 2013; Jung and Lee 2012; Lee et al. 2016), although MRS10 was more sensitive than IBA3. Contrarily to copper, cadmium is a non-essential metal and presents high toxicity at low concentrations (Kang et al. 2018), being very dispersed in the aquatic environment and, due to its high bioavailability, it is easily transferred along the food chain. IBA3 was significantly more tolerant to cadmium than MRS10 (210.5 mg L−1 and 130.8 mg L−1, respectively) (Table 1). In a study by Kang et al. (2019), the LC50 value obtained for neonates of B. koreanus (142.69 mg L−1) was similar to the one obtained for MRS10 but for only 6 h of exposure. This seems to indicate that MRS10 and IBA3 have higher tolerance to cadmium than the organisms studied by Kang et al. (2019). These authors observed that body size correlated inversely with metabolic rate, which is a key factor for cadmium bioaccumulation. They also showed a body size-dependent interspecific tolerance, with the smaller B. rotundiformis presenting the lowest 6 h-LC50 value and the larger B. plicatilis the highest (123.33 and 309.34 mg L−1, respectively) when exposing neonates. Also, for B. koreanus, the LC50 value increased from 142.69 mg L−1 in neonates to 306.07 mg L−1 when exposing adults. In fact, IBA3 present larger body length than MRS10 during the initial developmental stages (Granada et al. 2022), which can explain its higher tolerance to this stressor.
Although prohibited in many countries for its adverse health effect in humans, the antibiotic chloramphenicol is still used in aquaculture and animal farming in some developing countries, due to its broad-spectrum antimicrobial activity and low cost (Romero-Soto et al. 2018). It is also used in antibiotic cocktails to eliminate rotifer-associated bacteria and obtain axenic cultures (Martínez-Díaz et al. 2003). Besides antibiotics, hydrogen peroxide is a therapeutant used to prevent and control mortality caused by external fungal infections in cultured fish and their eggs, having the potential to also control external bacterial infections and parasitic infections. The use of hydrogen peroxide in aquaculture and its effects on freshwater and brackish water organisms was reviewed by Schmidt et al. (2006). In mass productions, rotifers grow in association with a complex bacterial ecosystem, and these bacteria can improve the culture by constituting food for rotifers or producing compounds (e.g. vitamin B12) for growth support. However, these bacteria can be harmful for fish larvae, compromising their performance and survival (Martínez-Díaz et al. 2003). Therefore, it is recommended to eliminate the rotifer-associated bacteria before using rotifers as live food. In addition, axenic rotifers are particularly useful for nutritional science, probiotic, molecular biology, development of immune system, and genetic studies (Suga et al. 2011). In this study, when exposed to hydrogen peroxide, MRS10 were more tolerant than IBA3 (LC50 of 2.42 mg L−1 and 2.27 mg L−1, respectively), contrarily to what was observed for the other stressors (Table 1). In another study, exposure to 3% hydrogen peroxide for 6–10 min showed to be effective in eliminating rotifer-associated bacteria in B. plicatilis, although rotifer mortality and unviability of amictic eggs were also observed at these conditions (Martínez-Díaz et al. 2003). Concentrations much lower than 0.1% were extremely harmful for both strains in the present study.
Regarding chloramphenicol, no effects on survival were observed for any of the two strains of B. koreanus, even at the higher concentration tested (100 mg L−1). Martínez-Díaz et al. (2003) have also observed no effects on survival of B. plicatilis when the rotifers were exposed to an antibiotic cocktail, with chloramphenicol at a maximum concentration of 40 mg L−1. Although no effects on survival were seen in those studies, Suga et al. (2011) showed that when exposing B. plicatilis to 40 mg L−1 of chloramphenicol, the antibiotic had a toxic effect on germ cell and egg development, along with innate malformations in F2 generation and abnormal sterile neonates. This highlights the increasing importance of studying sub-lethal effects of a substance since the non-existence of lethal effects does not necessarily imply it will not be harmful at other levels.
Swimming behaviour as a more sensitive endpoint for effect assessment between strains
The movement of Brachionus spp. comprises periods of free swimming and periods of attachment to substrata with the pedal gland (Kim et al. 2020). The helical swimming, characteristic of rotifers, results from the coordinated beat of cilia that is controlled by two innerved muscles located in the infraciliature (Charoy and Janssen 1999). For rotifers, swimming is a high energy demand action, highly affected by intrinsic (e.g. female age and body size) and extrinsic factors (Yúfera et al. 2005). Since metabolic activity can compromise cilia beating ability and/or its coordination, leading to alterations in the swimming pattern, studies have been using swimming speed as a parameter to evaluate the physiological condition of rotifers and the status of rotifer mass cultures (Kim et al. 2020; Korstad et al. 1995). A decrease in not only swimming speed but also in swimming frequency is usually observed in rotifers under physical stress, namely when the organisms are trying to maintain the osmotic equilibrium, given that the attachment may be a way to conserve energy and help rotifers to cope with stressful conditions (Kim et al. 2020). In the present work, the endpoint studied was the swimming capacity inhibition by measuring rotifer swimming speed under several conditions, with the aim of comparing differences in sensitivity between strains.
In general, a dose-depended significant inhibition of swimming capacity was observed for all stressors except chloramphenicol. IBA3 showed to be more tolerant to low and high salinities, while MRS10 was more tolerant to metals. There were no differences between strains for hydrogen peroxide. For stressors such as high salinity and cadmium chloride, the swimming inhibition was mainly due to a decrease in speed. However, in organisms exposed to copper sulfate and, especially, to hydrogen peroxide, this decrease was mainly due to high attachment, which resulted in null speed values. In further studies with behavioural endpoints, it would be interesting to analyse effects not only on swimming speed, but also in swimming impairment by calculating attachment rates.
Overall, results obtained for swimming capacity did not reflect the ones observed in survival. Similar tendency between lethal and sub-lethal tests was only observed for low salinity, being IBA3 more tolerant in both situations. For both metals studied, MRS10 was more sensitive in terms of survival, but more tolerant when evaluating swimming capacity. High salinity had the same effect on strains’ survival, but lower effect in IBA3 behaviour; while MRS10 showed to be more tolerant to hydrogen peroxide at the survival level, but no differences in tolerance were observed in behaviour for this stressor. Although there are no genetic and morphological differences between strains, MRS10 strain starts reproducing at smaller sizes than IBA3 strain, and IBA3 has a longer life span (Granada et al. 2022). Foley et al. (2019) indicated several studies, including with invertebrates, where lifespan and stress resistance have shown to be positively related, and studies where extended lifespan and antioxidant capacity are strongly associated. This may explain the fact that IBA3 was, in general, more tolerant than MRS10.
Contrariwise to the other stressors, the lethal endpoint was more sensitive than the swimming behaviour after exposure to cadmium chloride (Table 2), with MRS10 showing a considerably higher value of 6 h-EC50 (272.14 mg L−1 vs 130.8 mg L−1), and a hormetic response (Fig. 3). This type of response was also observed for hydrogen peroxide for both strains, meaning that at low toxicity levels, rotifers were overcompensating the homeostasis disruption, presenting a response curve with a low-dose stimulation (Liang et al. 2018). This type of response occurs with a broad range of chemicals (Calabrese and Baldwin 1998), and was already observed in rotifers, more commonly when exposed to essential elements (Rebolledo et al. 2020).
Swimming behaviour showed to be especially sensitive to assess the effect of high salinity, hydrogen peroxide, and copper sulfate. As most of marine invertebrates, B. plicatilis has been considered an osmoconformer, meaning it keeps internal fluids isotonic to the environment (Fielder et al. 2000; Talley and Talley 2008). However, Lowe et al. (2005) observed patterns of Na+/K+ ATPase activity in B. plicatilis in response to salinity, suggestive of osmoregulation, being the expend of more energy on the osmotic regulation an explanation for the fact that at higher salinities (30–60 psu), in different studies, a decrease in growth rate and amictic egg production was observed (Lowe et al. 2005; Montero-Pau et al. 2011). This could explain why, at high salinities (40–80 psu), swimming capacity tests had to be performed with lower values than acute tests, as possible to observe by the difference between 24 h-LC50 and 6 h-EC50 values (Tables 1 and 2), especially for MRS10 (70.13 psu vs 49.55 psu). For hydrogen peroxide, the lowest concentration used for 24 h lethal tests was used as the highest concentration for 6 h sub-lethal exposure. And for copper sulfate, it was possible to observe sub-lethal effects at concentrations ten times lower than in the lethal test (0.0877 mg L−1 vs 0.81 mg L−1, respectively, for IBA3). Likewise, Garaventa et al. (2010) could detect alterations in swimming speed of B. plicatilis when exposed to concentrations that were less than 5% of LC50 values of three toxic substances; and the swimming behaviour of B. koreanus was significantly impacted by a disinfectant in concentrations lower than the lethal NOEC values (Won et al. 2022).
Chronic tests have been proposed as alternatives to lethal tests in rotifers, due to their ecological relevance (Rebolledo et al. 2020), with emphasis on behaviour endpoints (e.g. swimming behaviour), as they allow a faster assessment of effects (Dahms et al. 2011). Moreover, Won et al. (2022) showed swimming behaviour was not only more sensitive than lethal exposures, but also more sensitive than other important and more time consuming chronic endpoints, such as life table parameters. When exposing B. koreanus to concentrations lower than the lethal NOEC values of one toxic substance, these authors observed alterations in both swimming speed and movement patterns of rotifers, while the same conditions did not significantly changed life table parameters (Won et al. 2022). Likewise, in the present study, swimming behaviour was a good alternative to lethal endpoints to assess the effect of several stressors in rotifers. Moreover, it was possible to detect tolerance differences between strains for several types of stressors, with shorter exposures than in lethal tests and, for some of the stressors, with lower concentrations.
Conclusion
In this study, two strains of B. koreanus were exposed to several abiotic stressors. The fact that one strain was generally more tolerant than the other, although they belong to the same species and are maintained under the same conditions for several years, evidenced the importance of knowing the characteristics of the water body of origin and to perform multiclonal experiments. The results also highlighted the potential of swimming capacity inhibition as a toxicity endpoint to assess differences in response between rotifer strains, being more sensitive and of shorter duration than lethal assays.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Calabrese EJ, Baldwin LA (1998) Hormesis as a biological hypothesis, in: Environmental Health Perspectives. Public Health Services, US Dept of Health and Human Services, pp. 357–362. https://doi.org/10.1289/ehp.98106s1357
Campillo S, García-Roger EM, Carmona MJ, Serra M (2011) Local adaptation in rotifer populations. Evol Ecol 25:933–947. https://doi.org/10.1007/s10682-010-9447-5
Charoy C, Janssen CR (1999) The swimming behaviour of Brachionus calyciflorus (rotifer) under toxic stress. II. Comparative sensitivity of various behavioural criteria. Chemosphere 38:3247–3260. https://doi.org/10.1016/S0045-6535(98)00557-8
Chen J, Wang Z, Li G, Guo R (2014) The swimming speed alteration of two freshwater rotifers Brachionus calyciflorus and Asplanchna brightwelli under dimethoate stress. Chemosphere 95:256–260. https://doi.org/10.1016/j.chemosphere.2013.08.086
Ciros-Pérez J, Gómez A, Serra M (2001) On the taxonomy of three sympatric sibling species of the Brachionus plicatilis (Rotifera) complex from Spain, with the description of B. ibericus n. sp. J Plankton Res 23:1311–1328. https://doi.org/10.1093/plankt/23.12.1311
Cooper CA, Tait T, Gray H, Cimprich G, Santore RC, McGeer JC, Wood CM, Smith DS (2014) Influence of salinity and dissolved organic carbon on acute Cu toxicity to the rotifer Brachionus plicatilis. Environ Sci Technol 48:1213–1221. https://doi.org/10.1021/es402186w
Dahms HU, Hagiwara A, Lee JS (2011) Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat Toxicol 101:1–12. https://doi.org/10.1016/j.aquatox.2010.09.006
Fielder DS, Purser GJ, Battaglene SC (2000) Effect of rapid changes in temperature and salinity on availability of the rotifers Brachionus rotundiformis and Brachionus plicatilis. Aquaculture 189:85–99. https://doi.org/10.1016/S0044-8486(00)00369-0
Foley HB, Sun PY, Ramirez R, So BK, Venkataraman YR, Nixon EN, Davies KJA, Edmands S (2019) Sex-specific stress tolerance, proteolysis, and lifespan in the invertebrate Tigriopus californicus. Exp Gerontol 119:146–156. https://doi.org/10.1016/j.exger.2019.02.006
Forberg T (2013) Larvi 2013. Aquaculture.Ugent.Be 2–5
Garaventa F, Gambardella C, Di Fino A, Pittore M, Faimali M (2010) Swimming speed alteration of Artemia sp. and Brachionus plicatilis as a sub-lethal behavioural end-point for ecotoxicological surveys. Ecotoxicology 19:512–519. https://doi.org/10.1007/s10646-010-0461-8
Gómez A, Carmona MJ, Serra M (1997) Ecological factors affecting gene flow in the Brachionus plicatilis complex (Rotifera). Oecologia 111:350–356. https://doi.org/10.1007/s004420050245
Granada L, Lemos MFL, Bossier P, Novais SC (2022) Genetic identification and comparative study on life history parameters of two strains belonging to Brachionus plicatilis species complex (Rotifera: Monogononta). Aquacult Res 26:101309. https://doi.org/10.1016/j.aqrep.2022.101309
Gribble KE, Jarvis G, Bock M, Mark Welch DB (2014) Maternal caloric restriction partially rescues the deleterious effects of advanced maternal age on offspring. Aging Cell 13:623–630. https://doi.org/10.1111/acel.12217
Hagiwara A, Kotani T, Snell TW, Assava-Aree M, Hirayama K (1995) Morphology, reproduction, genetics, and mating behavior of small, tropical marine Brachionus strains (Rotifera). J Exp Mar Biol Ecol 194:25–37. https://doi.org/10.1016/0022-0981(95)00081-X
Han C, Kim HJ, Lee JS, Hagiwara A (2023) Differential modes of response to zinc chloride in the marine rotifers Brachionus plicatilis and Brachionus rotundiformis: reproductive flexibility and stress-defensive activity. Aquaculture 564:739081. https://doi.org/10.1016/J.AQUACULTURE.2022.739081
Han, J., Lee, K.W., 2020. Influence of salinity on population growth, oxidative stress and antioxidant defense system in the marine monogonont rotifer Brachionus plicatilis. Comparative Biochemistry and Physiology - Part B: Biochemistry and Molecular Biology 250, 110487. https://doi.org/10.1016/j.cbpb.2020.110487
Han J, Won EJ, Hwang DS, Rhee JS, Kim IC, Lee JS (2013) Effect of copper exposure on GST activity and on the expression of four GSTs under oxidative stress condition in the monogonont rotifer, Brachionus koreanus. Comparative Biochem Physiol - Part c: Toxicol Pharmacol 158:91–100. https://doi.org/10.1016/j.cbpc.2013.05.006
Hwang DS, Dahms HU, Park HG, Lee JS (2013) A new intertidal Brachionus and intrageneric phylogenetic relationships among Brachionus as revealed by allometry and CO1-ITS1 gene analysis. Zool Stud 52:13. https://doi.org/10.1186/1810-522X-52-13
ISO 19820:2016(E), Water quality — determination of the acute toxicity to the marine rotifer Brachionus plicatilis
Jeong CB, Lee YH, Park JC, Kang HM, Hagiwara A, Lee JS (2019) Effects of metal-polluted seawater on life parameters and the induction of oxidative stress in the marine rotifer Brachionus koreanus. Comparative Biochemistry and Physiology – Part C: Toxicology and Pharmacology 225. https://doi.org/10.1016/j.cbpc.2019.108576
Joshi PS (1988) Influence of salinity on population growth of a Rotifer, Brachionus plicatilis (Mullen). J Indian Fish Assoc 18:75–81
Jung M-Y, Lee Y-M (2012) Expression profiles of heat shock protein gene families in the monogonont rotifer Brachionus koreanus — exposed to copper and cadmium. Toxicol Environ Heal Sci 4:235–242. https://doi.org/10.1007/s13530-012-0141-6
Kaneko G, Yoshinaga T, Yanagawa Y, Ozaki Y, Tsukamoto K, Watabe S (2011) Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Funct Ecol 25:209–216. https://doi.org/10.1111/j.1365-2435.2010.01773.x
Kang HM, Jeong CB, Kim MS, Lee JS, Zhou J, Lee YH, Kim DH, Moon E, Kweon HS, Lee SJ, Lee JS (2018) The role of the p38-activated protein kinase signaling pathway-mediated autophagy in cadmium-exposed monogonont rotifer Brachious koreanus. Aquat Toxicol 194:46–56. https://doi.org/10.1016/j.aquatox.2017.11.002
Kang HM, Lee JS, Lee YH, Kim MS, Park HG, Jeong CB, Lee JS (2019) Body size-dependent interspecific tolerance to cadmium and their molecular responses in the marine rotifer Brachionus spp. Aquat Toxicol 206:195–202. https://doi.org/10.1016/j.aquatox.2018.10.020
Kim HJ, Ohtani M, Kakumu A, Sakakura Y, Hagiwara A (2020) External factors that regulate movement in the marine rotifer Brachionus plicatilis. Fish Sci 86:655–663. https://doi.org/10.1007/s12562-020-01438-w
Korstad J, Neyts A, Danielsen T, Overrein I, Olsen Y (1995) Use of swimming speed and egg ratio as predictors of the status of rotifer cultures in aquaculture. Hydrobiologia 313–314:395–398. https://doi.org/10.1007/BF00025976
Kostopoulou V, Carmona MJ, Divanach P (2012) The rotifer Brachionus plicatilis: an emerging bio-tool for numerous applications. J Biol Res 17:97–112
Lee JW, Kang HM, Won EJ, Hwang DS, Kim DH, Lee SJ, Lee JS (2016) Multi-walled carbon nanotubes (MWCNTs) lead to growth retardation, antioxidant depletion, and activation of the ERK signaling pathway but decrease copper bioavailability in the monogonont rotifer (Brachionus koreanus). Aquat Toxicol 172:67–79. https://doi.org/10.1016/j.aquatox.2015.12.022
Lee MC, Park JC, Kim DH, Kang S, Shin KH, Park HG, Han J, Lee JS (2017) Interrelationship of salinity shift with oxidative stress and lipid metabolism in the monogonont rotifer Brachionus koreanus. Comp Biochem Physiol A Mol Integr Physiol 214:79–84. https://doi.org/10.1016/j.cbpa.2017.09.014
Li XD, Wang XY, Xu ME, Jiang Y, Yan T, Wang XC (2020) Progress on the usage of the rotifer Brachionus plicatilis in marine ecotoxicology: a review. Aquatic Toxicol. https://doi.org/10.1016/j.aquatox.2020.105678
Liang Y, Lu X, Min Y, Liu L, Yang J (2018) Interactive effects of microcystin and ammonia on the reproductive performance and phenotypic traits of the rotifer Brachionus calyciflorus. Ecotoxicol Environ Saf 147:413–422. https://doi.org/10.1016/J.ECOENV.2017.08.070
Lowe CD, Kemp SJ, Bates AD, Montagnes DJS (2005) Evidence that the rotifer Brachionus plicatilis is not an osmoconformer. Mar Biol 146:923–929. https://doi.org/10.1007/s00227-004-1501-9
Lubzens E (1987) Raising rotifers for use in aquaculture. Hydrobiologia 147:245–255. https://doi.org/10.1007/BF00025750
Marcial HS, Hagiwara A, Snell TW (2005) Effect of some pesticides on reproduction of rotifer Brachionus plicatilis Müller. Hydrobiologia 546:569–575. https://doi.org/10.1007/s10750-005-4302-3
Martínez-Díaz SF, Álvarez-González CA, Legorreta MM, Vázquez-Juárez R, Barrios-González J (2003) Elimination of the associated microbial community and bioencapsulation of bacteria in the rotifer Brachionus plicatilis. Aquacult Int 11:95–108. https://doi.org/10.1023/A:1024117109362
Melvin SD, Wilson SP (2013) The utility of behavioral studies for aquatic toxicology testing: a meta-analysis. Chemosphere 93:2217–2223. https://doi.org/10.1016/j.chemosphere.2013.07.036
Montero-Pau J, Ramos-Rodríguez E, Serra M, Gómez A (2011) Long-term coexistence of rotifer cryptic species. PLoS One 6:e21530. https://doi.org/10.1371/journal.pone.0021530
Papakostas S, Dooms S, Christodoulou M, Triantafyllidis A, Kappas I, Dierckens K, Bossier P, Sorgeloos P, Abatzopoulos TJ (2006) Identification of cultured brachionus rotifers based on RFLP and SSCP screening. Kathmandu Univ Med J 4:470–473. https://doi.org/10.1007/s10126-005-6181-z
Pérez-Legaspi IA, Rico-Martínez R (2001) Acute toxicity tests on three species of the genus Lecane (Rotifera: Monogononta). Hydrobiologia 446(447):375–381. https://doi.org/10.1023/A:1017531712808
Rebolledo UA, Nandini S, Sarma SSS, Escobar-Sánchez O (2020) Effect of salinity and temperature on the acute and chronic toxicity of arsenic to the marine rotifers Proales similis and Brachionus ibericus. Marine Pollut Bull 157:111341. https://doi.org/10.1016/j.marpolbul.2020.111341
Rico-Martínez R, Arzate-Cárdenas MA, Robles-Vargas D, Pérez-Legaspi IA, Jesús A, Santos-Medrano GE (2016) Rotifers as models in toxicity screening of chemicals and environmental samples. Invertebrates - Experimental Models in Toxicity Screening 57–99. https://doi.org/10.5772/61771
Rico-Martínez R, Pérez-Legaspi IA, Arias-Almeida JC, Santos-Medrano GE (2013) Rotifers in ecotoxicology, in: Férard, J.F., Blaise, C. (Eds.), Encyclopedia of Aquatic Ecotoxicology. Springer, Dordrecht, pp. 973–996. https://doi.org/10.1007/978-94-007-5704-2_89
Romero-Soto IC, Dia O, Leyva-Soto LA, Drogui P, Buelna G, Díaz-Tenorio LM, Ulloa-Mercado RG, Gortáres-Moroyoqui P (2018) Degradation of chloramphenicol in synthetic and aquaculture wastewater using electrooxidation. J Environ Qual 47:805–811. https://doi.org/10.2134/jeq2017.12.0475
Schmidt LJ, Gaikowski MP, Gingerich WH (2006) Environmental assessment for the use of hydrogen peroxide in aquaculture for treating external fungal and bacterial diseases of cultured fish and fish eggs, United States Geological Survey, Biological Resources Division Upper, Upper Midwest Environmental 54603, 11545–11550. https://doi.org/10.1073/pnas.1411607111
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods. https://doi.org/10.1038/nmeth.2089
Serra M, Fontaneto D (2017) Speciation in the Brachionus plicatilis species complex, in: Hagiwara, A., Yoshinaga, T. (Eds.), Rotifers. Springer Nature Singapore Pte Ltd. and the Japanese Society of Fisheries Science, pp. 15–32. https://doi.org/10.1007/978-981-10-5635-2_2
Snare DJ, Fields AM, Snell TW, Kubanek J (2013) Lifespan extension of rotifers by treatment with red algal extracts. Exp Gerontol 48:1–17. https://doi.org/10.1016/j.exger.2013.09.007.Lifespan
Snell TW, Janssen CR (1995) Rotifers in ecotoxicology: a review. Hydrobiologia 313–314:231–247. https://doi.org/10.1007/BF00025956
Suga K, Tanaka Y, Sakakura Y, Hagiwara A (2011) Axenic culture of Brachionus plicatilis using antibiotics. Hydrobiologia 662:113–119. https://doi.org/10.1007/s10750-010-0488-0
Talley DM, Talley TS (2008) Salinity, in: Jørgensen, S.E., Fath, B.D. (Eds.), Encyclopedia of ecology, five-volume set. Elsevier Science, pp. 3127–3132. https://doi.org/10.1016/B978-008045405-4.00541-3
Won EJ, Byeon E, Lee YH, Jeong H, Lee Y, Kim MS, Jo HW, Moon JK, Wang M, Lee JS, Shin KH (2022) Molecular evidence for suppression of swimming behavior and reproduction in the estuarine rotifer Brachionus koreanus in response to COVID-19 disinfectants. Marine Pollut Bull 175, 113396. https://doi.org/10.1016/J.MARPOLBUL.2022.113396
Yoon SJ, Park GS (2011) Ecotoxicological effects of brine discharge on marine community by seawater desalination. Desalin Water Treat 33:240–247. https://doi.org/10.5004/dwt.2011.2644
Yúfera M (2001) Studies on Brachionus (Rotifera): an example of interaction between fundamental and applied research, in: Hydrobiologia. pp. 383–392. https://doi.org/10.1023/A:1017583729646
Yúfera M, Pascual E, Olivares JM (2005) Factors affecting swimming speed in the rotifer Brachionus plicatilis, in: Hydrobiologia. pp. 375–380. https://doi.org/10.1007/s10750-005-4279-y
Funding
Open access funding provided by FCT|FCCN (b-on). This study had the support of Fundação para a Ciência e a Tecnologia (FCT) through the Strategic Project granted to MARE – Marine and Environmental Sciences Centre (UIDB/04292/2020 and UIDP/04292/2020) and the project granted to the Associated Laboratory ARNET (LA/P/0069/2020). Luana Granada also wish to acknowledge the financial support given by FCT (SFRH/BD/102036/2014). Sara Novais is funded by national funds through FCT, in the scope of the framework contract foreseen in the numbers 4, 5, and 6 of the article 23, of the Decree-Law 57/2016, of 29 August, changed by Law 57/2017, of 19 July.
Author information
Authors and Affiliations
Contributions
Luana Granada: Conceptualization, Methodology, Formal analysis, Investigation, Writing—original draft preparation, Visualization. Marco F.L. Lemos: Resources, Writing—review & editing, Funding acquisition. Peter Bossier: Resources, Writing—review & editing, Supervision. Sara C. Novais: Conceptualization, Methodology, Resources, Writing—review & editing, Validation, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Invertebrate animals used for this study do not require specific approval from the ethical committee.
Consent for publication
This manuscript is an original work that has not been previously published whole or in part, and is not under consideration for publication elsewhere. All authors agreed that the work is ready for submission to this journal, and accept responsibility for the manuscript’s contents.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Granada, L., Lemos, M.F.L., Bossier, P. et al. Swimming behaviour as an alternative endpoint to assess differences in abiotic stress sensitivities between strains of Brachionus koreanus (Rotifera: Monogononta). Environ Sci Pollut Res 30, 56137–56147 (2023). https://doi.org/10.1007/s11356-023-26190-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26190-3