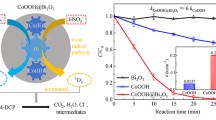
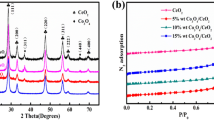
Abstract
Although heterogeneous cobalt-based catalysts have been widely studied and used in SO4•− based advanced oxidation processes, the efficiencies were still not high enough due to the limiting step of Co(III)/Co(II) cycle in the system. In this study, a bimetallic oxide composed of Co and Mo was designed and used for enhancing the performance of peroxymonosulfate activation on organic pollutants removal. The CoMoO4 nanorods exhibited superior catalytic activity for methylene blue (MB) degradation than Co3O4, MoO3, and their mechanical mixture, which was attributed to the synergetic effect between Co and Mo. CoMoO4 nanorods were able to efficiently degrade MB under a wide pH range of 3–11 and could maintain high efficiency in 5 cycles with less leakage of metal ions. Moreover, CoMoO4 nanorods displayed broad spectrum applicability to the different water matrix and a variety of pollutants such as phenol and Congo red. The Co(II) was proved to be the main active site of the catalyst, while Mo played an important role in promoting the Co(III)/Co(II) cycle. Surface free radicals are the main active species in the degradation process. This work provides new insights into the design of cobalt-based bimetallic catalyst and the improvement on PMS activation.











Similar content being viewed by others
Data availability
The datasets and materials used in study are available from authors.
References
Ahmad I, Fasihullah Q, Vaid FHM (2005) Effect of phosphate buffer on photodegradation reactions of riboflavin in aqueous solution. J Photochem Photobiol B Biol 78(3):229–234
Ahmadi M, Ghanbari F (2019) Organic dye degradation through peroxymonosulfate catalyzed by reusable graphite felt/ferriferrous oxide: mechanism and identification of intermediates. Mater Res Bull 111:43–52
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38(13):3705–3712
Chen J, Zhang P (2006) Photodegradation of perfluorooctanoic acid in water under irradiation of 254 nm and 185 nm light by use of persulfate. Water Sci Technol 54:317–325
Chen X, Chen J, Qiao X, Wang D, Cai X (2008) Performance of nano-Co3O4/peroxymonosulfate system: kinetics and mechanism study using acid orange 7 as a model compound. Appl Catal B 80:116–121
Deng J, Feng SF, Zhang K, Li J, Ma X (2017) Heterogeneous activation of peroxymonosulfate using ordered mesoporous Co3O4 for the degradation of chloramphenicol at neutral pH. Chem Eng J 308:505–515
Du YC, Ma WJ, Liu PX, Zou BH, Ma J (2016) Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J Hazard Mater 308:58–66
Fan YN, Ma WJ, He JL, Du YC (2017) CoMoO4 as a novel heterogeneous catalyst of peroxymonosulfate activation for the degradation of organic dyes. RSC Adv 7:36193–36200
Ghanbari F, Wang Q, Hassani A, Wacławek S, Rodríguez-Chueca J, Lin KYA (2021) Electrochemical activation of peroxides for treatment of contaminated water with landfill leachate: efficacy, toxicity and biodegradability evaluation. Chemosphere 279:130610
Guo XJ, Wang KB, Li D, Qin JB (2017) Heterogeneous photo-Fenton processes using graphite carbon coating hollow CuFe2O4 spheres for the degradation of methylene blue. Applied Surface Science: A Journal Devoted to the Properties of Interfaces in Relation to the Synthesis and Behaviour of Materials 420:792–801
Han Q, Yang S, Xin Y, Shao X, Niu R, Wang L (2012) Cobalt catalyzed peroxymonosulfate oxidation: a review of mechanisms and applications on degradating organic pollutants in water. Prog Chem 24:144–156
Hassani A, Eghbali P, Metin Ö (2018) Sonocatalytic removal of methylene blue from water solution by cobalt ferrite/mesoporous graphitic carbon nitride (CoFe2O4/mpg-C3N4) nanocomposites: response surface methodology approach. Environ Sci Pollut Res 25(32):32140–32155
Hassani A, Eghbali P, Kakavandi B, Lin KYA, Ghanbari F (2020) Acetaminophen removal from aqueous solutions through peroxymonosulfate activation by CoFe2O4/mpg-C3N4 nanocomposite: insight into the performance and degradation kinetics. Environ Technol Innov 20:101127
He XX, Cruz AA, Hiskia A, Kaloudis T, Shea K, Dionysiou DD (2015) Destruction of microcystins (cyanotoxins) by UV-254nm-based direct photolysis and advanced oxidation processes (AOPs): influence of variable amino acids on the degradation kinetics and reaction mechanisms. Water Res 74:227–238
Hori H, Nagaoka Y, Murayama M, Kutsuna S (2008) Efficient decomposition of perfluorocarboxylic acids and alternative fluorochemical surfactants in hot water. Environ Sci Technol 42(19):7438–7443
Huang Z, Bao H, Yao Y, Lu J, Lu W, Chen W (2016) Key role of activated carbon fibers in enhanced decomposition of pollutants using heterogeneous cobalt/peroxymonosulfate system. J Chem Technol Biotechnol 91(5):1257–1265
Jiang X, Guo Y, Zhang L, Jiang W, Xie R (2018) Catalytic degradation of tetracycline hydrochloride by persulfate activated with nano Fe0 immobilized mesoporous carbon. Chem Eng J 178:392–401
Jing J, Pervez MN, Sun P, Cao C, Li B, Naddeo V, Jin W, Zhao Y (2021) Highly efficient removal of bisphenol A by a novel Co-doped LaFeO3 perovskite/PMS system in salinity water. Sci Total Environ 801:149490
Karim AV, Hassani A, Eghbali P, Nidheesh PV (2022) Nanostructured modified layered double hydroxides (LDHs)-based catalysts: a review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr Opin Solid State Mater Sci 26(1):100965
Laat DJ, Dao YH, Najjar NHE, Daou C (2011) Effect of some parameters on the rate of the catalysed decomposition of hydrogen peroxide by iron(III)-nitrilotriacetate in water. Water Res 45(17):5654–5664
Li W, Orozco R, Camargos N, Liu H (2017) Mechanisms on the impacts of alkalinity, pH, and chloride on persulfate-based groundwater remediation. Environ Sci Technol 51(7):3948–3959
Liu YN, Qu RX, Li XY, Wei Y, Feng L (2020a) A bifunctional β-MnO2 mesh for expeditious and ambient degradation of dyes in activation of peroxymonosulfate (PMS) and simultaneous oil removal from water. J Colloid Interface Sci 579:412–424
Liu Y, Huo XW, Zhou P, Liu YX, Cheng F, Cheng X, Zhang YL, Wang QG (2020b) Removal of contaminants by activating peroxymonosulfate (PMS) using zero valent iron (ZVI)-based bimetallic particles (ZVI/Cu, ZVI/Co, ZVI/Ni, and ZVI/Ag). RSC Adv 5:57444–57452
Liu L, Li YN, Li W, Zhong RX, Lan YQ, Guo J (2020c) The efficient degradation of sulfisoxazole by singlet oxygen (1O2) derived from activated peroxymonosulfate (PMS) with Co3O4–SnO2/RSBC. Environ Res 187:109665
Liu D, Chen D, Hao Z, Tang Y, Jiang L, Li T, Jia B (2022) Efficient degradation of rhodamine B in water by CoFe2O4/H2O2 and CoFe2O4/PMS systems: a comparative study. Chemosphere 307:135935
Ma J, Yang Y, Jiang X, Xie Z, Chen C, Chen H (2018) Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water. Chemosphere 190:296–306
Madihi-Bidgoli S, Asadnezhad S, Yaghoot-Nezhad A, Hassani A (2021) Azurobine degradation using Fe2O3@ multi-walled carbon nanotube activated peroxymonosulfate (PMS) under UVA-LED irradiation: performance, mechanism and environmental application. J Environ Chem Eng 9(6):106660
Nakanishi T, Maekawa H, Yokokawa T (2007) Redox potential of Mo(VI)/Mo in alkali borate melts, materials transactions. JIM 39(3):399–403
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284
Nguyen TB, Huang CP, Doong RA, Wang MH, Chen CW, Dong CD (2022) Manipulating the morphology of 3D flower-like CoMn2O4 bimetallic catalyst for enhancing the activation of peroxymonosulfate toward the degradation of selected persistent pharmaceuticals in water. Chem Eng J 436:135244
Qi F, Chu W, Xu B (2014) Modeling the heterogeneous peroxymono-sulfate/Co-MCM41 process for the degradation of caffeine and the study of influence of cobalt sources. Chem Eng J 235:10–18
Rao CNR, Sood AK, Voggu R, Subrahmanyam KS (2010) Some novel attributes of graphene. The Journal of Physical Chemistry Letters 1(2):572–580
Tao X, Wu Y, Wu Y, Zhang B, Sha H, Cha L, Liu N (2018) Activated carbon-supported cobalt molybdate as a heterogeneous catalyst to activate peroxymonosulfate for removal of organic dyes. Appl Organomet Chem 32(12):e4572
Tong M, Liu F, Dong Q, Liu W (2020) Magnetic Fe3O4-deposited flower-like MoS2 nanocomposites for the Fenton-like Escherichia coli disinfection and diclofenac degradation. Journal of Hazardous Material 385:121604
Waldemer RH, Tratnyek PG, Johnson RL, Nurmi JT (2007) Oxidation of chlorinated ethenes by heat-activated persulfate:kinetics and products. Environ Sci Technol 41(3):1010–1015
Wang C, Zhao J, Chen C, Na P (2021) Catalytic activation of PS/PMS over Fe-Co bimetallic oxides for phenol oxidation under alkaline conditions. Appl Surf Sci 562:150134
Williams G, Kamat PV (2009) Graphene-semiconductor nanocomposites: excited-state interactions between ZnO nanoparticles and graphene oxide. Langmuir 25(24):13869–13873
Xu HD, Zhang YZ, Li JJ, Hao QQ (2019) Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols. Environ Pollut 257(3):113610
Yong F, Liu JH, Wu DL, Zhou ZY, Deng Y, Zhang T, Shih K (2015) Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation. Chem Eng J 280:514–524
Yuan RX, Jiang ML, Gao SM, Wang ZH, Wang HY, Boczkaj G, Liu ZJ, Li ZJ (2020) 3D mesoporous α-Co(OH)2 nanosheets electrodeposited on nickel foam: a new generation of macroscopic cobalt-based hybrid for peroxymonosulfate activation. Chem Eng J 380:122427
Zhang B, Zhang Y, Xiang W, Teng Y, Wang Y (2017) Comparison of the catalytic performances of different commercial cobalt oxides for peroxymonosulfate activation during dye degradation. Chem Res Chin Univ 33(5):822–827
Zhao Y, Lu D, Xu C, Zhong J, Chen M, Xu S, Cao Y, Zhao Q, Yang M, Ma J (2020) Synergistic oxidation-filtration process analysis of catalytic CuFe2O4-tailored ceramic membrane filtration via peroxymonosulfate activation for humic acid treatment. Water Res 171:115387
Zhen J, Zhang S, Zhuang X, Ahmad S, Ni SQ (2021) Sulfate radicals based heterogeneous peroxymonosulfate system catalyzed by CuO-Fe3O4-Biochar nanocomposite for bisphenol A degradation. J Water Process Eng 41:102078
Zhu MP, Yang JCE, Duan X, Wang S, Fu ML (2020) Engineered Co2AlO4/CoAl2O4@Al2O3 monolithic catalysts for peroxymonosulfate activation: Co3+/Co2+ and ODefect/OLattice ratios dependence and mechanism. Chem Eng J 409:128162
Funding
This work was financially supported by the “Jie Bang Gua Shuai” of Science and technology Projects of Shenyang City in 2022, (22-316-3-11); the Natural Science Foundation of Liaoning province (No.2023-MS-176).
Author information
Authors and Affiliations
Contributions
Conceptualization, writing and review by Huanxin Zhao, performing experiments by Wenkai Chen, writing and editing the original draft by Dan Wu, validation and formal analysis by Xinyue Liu, Wanjie Hu, and Xuejun Zhang. All of the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publish the paper upon acceptance.
Conflict of interest
The authors declare competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, H., Chen, W., Wu, D. et al. Coupling the effect of Co and Mo on peroxymonosulfate activation for the removal of organic pollutants. Environ Sci Pollut Res 30, 48389–48400 (2023). https://doi.org/10.1007/s11356-023-25755-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25755-6