Abstract
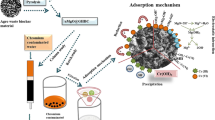
This study involved preparation and modification of Saccharum officinarium as adsorbent used for the removal of chromium (VI) ions in a batch process. The adsorbent was modified with oxalic acid for improved performance of the adsorbent by increasing the surface area of the adsorbent. Surface morphology of the adsorbents was characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD), while Fourier transform infrared (FT-IR) analysis was carried out before and after the adsorption of Cr (VI) ions to determine the participating functional group in the processes. The optimum adsorption was attained at pH 2 and contact time of 180 min with efficiency of adsorption of 56.7 and 92.6% onto RSO and MSO, respectively. The adsorption capacity increases with increase in initial metal ion concentration of the sorption mixture. The isotherms studies indicate that the experimental data were best fitted to Freundlich, Langmuir, and Sips models with R2 = 0.999 for adsorption of Cr (VI) ions onto raw S. officinarium (RSO) and modified S. officinarium (MSO). The maximum adsorption capacity obtained were 227.27 and 243.90 mg*g−1 while the adsorption energy obtained from D-R were found to be 3.460 and 6.325 kJ*mol−1 onto RSO and MSO, respectively. This revealed that the physiosorption process was favored in interaction of Cr (VI) ions onto both adsorbents. Separation factors obtained showed that the process is favored with increase in initial concentration of the adsorbate. Thermodynamic parameter values obtained showed that the sorption of Cr (VI) ions onto RSO and MSO is feasible, spontaneous, and endothermic in nature. The positive value of ΔS° indicates increase in disorderliness of the adsorption process. Kinetic data achieved at different initial concentrations of adsorbate have been analyzed, and the mechanism of the reaction was also studied by intra-particle and Bangham kinetic model. Each of the models was tested with R2 ˃ 0.9, where pseudo-second-order is the best-fitted model and Bangham mechanism only fitted with adsorption of Cr (VI) ions onto RSO. The reusability potential of RSO and MSO contributes to the economic values and reliability of the adsorbents for removal of Cr (VI) ions from aqueous solution.










Similar content being viewed by others
Data availability
Not applicable.
References
Adebayo GB, Adegoke HI, Fauzeeyat S (2020) Adsorption of Cr(VI) ions onto goethite, activated carbon and their composite: kinetic and thermodynamic studies. Appl Water Sci 10:213
Adebisi JA, Agunsoye JO, Bello SA, Haris M, Ramakokovhu MM, Daramola MO, Hassan SB (2017) Extraction of silica from sugarcane bagasse, cassava periderm and maize stalk: proximate analysis and physico-chemical properties of waste. Waste Biomass Valor 10:617–629
Ahmed SA, EL-Roudi AM, Salem AAA (2015) Removal of Mn (II) from ground water by solid waste of sugar industry. J Environ Sci Technol 8(6):38–351
Akram M, Khan B, Imran M, Ahmad I, Ajaz H, Tahir M, Rabbani F, Kaleem I, Akhtar MN, Ahmad N, Shah NS (2019) Biosorption of lead by cotton shells powder: characterization and equilibrium modeling study. Int J Phytoremediation 21(2):138–144
Aksu Z (2001) Equilibrium and kinetic modeling of cadmium vulga. biosorption by C. vulgans in a batch system effect of temperature. Sep Purf Technol 21:285
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environmental and their toxicological effects on humans. Heliyon 6(9):e04691
Crini G, Lichtfouse E, Wilson L, Morin-Crini N (2018) Adsorption-oriented processes using conventional and non-conventional adsorbents for wastewater treatment. Green Adsorbents for Pollutant Removal. Environmental Chemistry for a Sustainable World Springer Cham 18:23–71
Duarte-Nass C, Rebolledo K, Valenzuela T, Kopp M, Jeison D, Rivas M, Azocar L, Torres-Aravena A, Ciudad G (2020) Application of microbe-induced carbonate precipitation for copper removal from copper-enriched waters: challenges to future industrial application. J Environ Manage 256:109938
Ebelegi AN, Ayawei N, Wankasi D (2020) Interpretation of adsorption thermodynamics and kinetics. Open J Phy Chem 10:166–182
El-Naggar NEA, El-khateeb AY, Ghoniem AA (2020) Innovative low-cost biosorption process of Cr6+ by Pseudomonas alcaliphila NEWG-2. Sci Rep 10:14043
Erhayem M, Al-Tohami F, Mohamed R, Ahmida K (2015) Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis leaves. Am J Anal Chem 6:1–10
Evans SK, Wesley ON, Nathan O, Moloto MJ (2019) Chemically purified cellulose and its nanocrystals from sugarcane baggase: isolation and characterization. Heliyon 5:e02635
Gorzin F, Abadi MMBR (2017) Adsorption of Cr (VI) from aqueous solution by adsorbent prepared from paper mill sludge: kinetics and thermodynamic studies. Adsorp Sci Techno 36(1–2):149–169
Hanafiah SFM, Salleh NFM, Ghafar NA, Shukri NM, Kamarudin NHN, Hapani N, Jusoh R (2020) Efficiency of coconut husk as agricultural adsorbent in removal of chromium and nickel ions from aqueous solution. IOP Conf Series: Earth Environ Sci 596:012048
Hao L, Liu Q, Li X, Du Z, Wang P (2014) A potentially low-cost modified sawdust (MSD) effective for rapid Cr(VI) and As(V) removal from water. RSC Adv 4:49569
Hasan SH, Singh KK, Prakash O, Talat M, Ho YS (2008) Removal of Cr(VI) from aqueous solutions using agricultural waste ‘maize bran.’ J Hazard Mater 152:356–365
Ho YS, Wase DAJ, Forster CF (1995) Batch nickel removal from aqueous solution by sphagnum moss peat. Water Res 29:1327–1332
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72
Jaiswal A, Verma A, Jaiswal P (2018) Detrimental effects of heavy metals in soil, plants and aquatic ecosystems and in humans. J Environ Path Toxicol Oncol 37(3):183–197
Jeon S, Ito M, Tabelin CB, Pongsumrankul R, Kitajima N, Park I, Hiroyoshi N (2018) Gold recovery from shredder kight fraction of E-waste recycling plant by floatation-ammonium thiosulfate leaching. Waste Manage 77:195–202
Khan T, Isa MH, Mustafa R, Yeek-Chia H, Baloo L, Abd Manan TS, Saeed MO (2016) Cr (VI) adsorption from aqueous solution by an agricultural waste based carbon. RSC Adv 6:56365–56374
Kurniawan SB, Ahmad A, Said NSM (2021) Macrophytes as wastewater treatment agents: nutrient uptake and potential of produced biomass utilization toward circular economy initiatives. Sci Total Environ 790:148219
Labied R, Benturki O, Hamitouche A, Donnot A (2018) Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): kinetic, equilibrium, and thermodynamic study. Adsorp Sci Technol 36(3–4):1066–1099
Li Y, Liu J, Yuan Q, Tang H, Yu F, Lv X (2016) A green adsorbent derived from banana peel for highly effective removal of heavy metal ions from water. RSC Adv 6:45041–45048
Mahmoud A, Hoadley AFA (2012) An evaluation of a hybrid ion exchange electrodialysis process in the recovery of heavy metals from simulated dilute industrial wastewater. Water Res 46(10):3364–3376
Mahmood ul-Hassan M, Yasin M, Yousra M, Ahmad R, Sarwar S (2018) Kinetics, isotherms and thermodynamic studies of lead, chromium, and cadmium bio-adsorption from aqueous solution onto Picea smithiana sawdust. Environ Sci Pollut Res 25:12570–12578
Mekonnen E, Yitbarek M, Soreta TR (2015) Kinetic and thermodynamic studies of the adsorption of Cr (VI) onto some selected local adsorbents. South Africa J Chem 68:45–52
Mishra V (2017) Modeling of batch sorber system: kinetic, mechanistic, and thermodynamic modeling. Appl Water Sc 7:3173–3180
Mohapatra RK, Parhi PK, Pandey S, Bindhani BK, Thatoi H, Panda CR (2019) Active and passive biosorption of Pb(II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: kinetics and isotherm studies. J Environ Manage 247:121–134
Mullick A, Moulik S, Bhattacharjee S (2017) Removal of hexavalent chromium from aqueous solutions by low-cost rice husk-based activated carbon: kinetic and thermodynamic studies. Ind Chem Eng 60(1):58–71
Muthumareeswaran MR, Alhoshan M, Agarwal GP (2017) Ultrafiltration membrane for effective removal of chromium ions from potable water. Sci Rep 7:41423
Nasseh N, Taghavi L, Barikbin B, Harifi-Mood AR (2017) The removal of Cr(VI) from aqueous solution by almond green hull waste material: kinetic and equilibrium studies. J Water Reuse Desalina 7(4):449–460
Ngah WW, Hanafiah MAKM (2008) Removal of heavy metal ions from wastewater by chemically modified plant-wastes as adsorbents: a review. Biores Technol 99(10):3935–3948
Park KH, Parhi PK, Kang NH (2012) Studies on removal of low content copper from the sea nodule aqueous solution using the cationic resin TP 207. Sep Sci Technol 47(10):1531–1541
Ren B, Zhang Q, Zhang X, Zhao L, Li H (2018) Biosorption of Cr (VI) from aqueous solution using dormant spores of Aspergillus niger. RSC Adv 8:38157
Rice EW, Baird RB, Eaton AD, Clescen LS (2012) Standard methods for the examination of water and wastewater.Am Public Health Association, American Water Works Association and Water Environment Federation. Washington, D.C., USA
Seraj F, Rahman T (2018) Heavy metals, metalloids, their toxic effect and living systems. Am J Plant Sci 9:2626–2643
Sun JX, Sun XF, Sun RC, Fowler P, Baird MS (2003) Inhomogeneity in the chemical structure of sugarcane bagasse lignin. J Agric Food Chem 51:6719–6725
Teh CY, Budiman PM, Shak KPY, Wu YY (2016) Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind Eng Chem Res 55(16):4363–4389
Teli NC, Bhalerao SA (2019) Biosorption of chromium (VI) from aqueous solution using unmodified and nitrilotriacetic acid modified leaves of Ficus Benghalensis L. Int J Rec Sci Res 10(02):31045–31053
Tumolo M, Ancona V, De Paola D, Losacco D, Campanale C, Massarelli C, Uricchio VF (2020) Chromium pollution in European water, sources, health risk and remediation strategies: an overview. Int J Environ Res Public Health 17(5438):1–24
Ucun H, Bayhan YK, Kaya Y, Cakici A, Algur OF (2002) Biosorption of chromium (vi) from aqueous solution by cone biomass of pinus sylvestris. Biores Tech 85(2):155–158
Vaddi DR, Gurugubelli TR, Koutavarapu R, Lee DY, Shim J (2022) Bio-stimulated adsorption of Cr (VI) from aqueous solution by groundnut shell activated carbon@al embedded material. Catalysts 12:290
Vaiopoulou E, Gikas P (2020) Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 254:126876
Weber WJ, Morris JC (1962) Advances in water pollution research: removal of biologically resistant pollutants from waste waters by adsorption. Proc Int Conf Water Pollut Symp Oxford: Pergamon Press 2: 231–266
Yusuff AS (2018) Optimization of adsorption of Cr (VI) from aqueous solution by Leucaena leucocephala seed shell activated carbon using design of experiment. Appl Water Sci 8:232
Acknowledgements
Authors are obliged to CSIR—Central Electrochemical Research Institute, Karaikudi, Tamil Nadu, India, for the provision of facilities to carry out the instrumentation analysis for the research work.
Author information
Authors and Affiliations
Contributions
O.K. Akiode carried out the draft, conception, and design of the work with the acquisition, analysis, or interpretation of data for the work. A. Adetoro was involved in the drafting of the work and revising it critically for important intellectual content. A.I. Anene was involved in revising it critically for important intellectual content. S.O. Afolabi was involved in the final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Y.A. Alli was involved in the final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
All authors agreed to participate in the publication of the research article in ESPR.
Consent for publication
It is to the consent of all the authors to publish this work in ESPR.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akiode, O.K., Adetoro, A., Anene, A.I. et al. Methodical study of chromium (VI) ion adsorption from aqueous solution using low-cost agro-waste material: isotherm, kinetic, and thermodynamic studies. Environ Sci Pollut Res 30, 48036–48047 (2023). https://doi.org/10.1007/s11356-023-25706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25706-1