Abstract
Both physicochemical and biological factors affect the degree of metal accumulation in crayfish tissues. The content of metals and correlations between the metal concentrations in different tissues and the total length of crayfish is suitable indicators of contamination of the aquatic environment. The aim of the study was to analyse the effect of age and sex of crayfish on the degree of accumulation of Ca, Cu, and Zn in the muscle and exoskeleton. A total of 100 individuals of the spiny-cheek crayfish (Faxonius limosus, Rafinesque, 1817) were caught from Głowińsk reservoir (Poland) in October 2019 using fyke nets. Metal concentrations were determined in freeze-dried samples of the abdominal muscle, exoskeleton, bottom sediment, and water using atomic absorption spectroscopy (AAS). Here, we show that the highest concentrations of Zn were found in the muscle of 4-year-old females, Cu in 3-year-old males, and Ca in 4-year-old males. Sex was a significant factor affecting the content of Ca in the muscle and Zn in the exoskeleton. Age was a significant factor affecting the content of Zn, Cu, and Ca in the muscle and Zn and Cu in the exoskeleton. The bioconcentration factor (BCF) of Zn and Cu in the muscle and exoskeleton of spiny-cheek crayfish was much higher from water than from sediments, unlike Ca. Furthermore, we found significant correlation for muscle between Zn and total length in 3-year-old females and 4-year-old males and between Cu and TL in 3-year-old males. Analysing the recommended daily intake (RDI) for the investigated minerals confirmed that the consumption of 100 g of spiny-cheek crayfish muscle could meet daily requirement for Zn up to 27.5%, for Ca in 12.4%, and over 100% for Cu. The conducted analyses confirmed that the consumption of crayfish meat was safe for the health of potential consumers in terms of the analysed metal content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metals entering the environment may accumulate in animal tissues as they travel along the food chain through the diet (biomagnification), leading to high concentrations in organisms at the end of the trophic chain (Balzani et al. 2021). Among metals, there are macro- and microelements essential for animals and humans. Unfortunately, it should be noted that potentially harmless metals, e.g. Zn or Cu, become toxic to the organism when certain concentrations are exceeded (Nędzarek et al. 2020).
It has been proposed that crayfish muscle can be considered by the food industry as an alternative source of raw material (Śmietana et al. 2021). According to these authors, the abdomen and chelae muscles can be a source of minerals, too. As confirmed by numerous studies, crabs, shrimps, crayfishes, and lobsters are a valuable source of macro- and microelements essential for humans, but also of toxic metals in the case of water pollution (Balzani et al. 2022; Barrento et al. 2009; Heidarieh et al. 2013; Nędzarek et al. 2020; Raissy et al. 2011). Yet what about the exoskeleton? Crayfish are frequently suggested as bioindicators for monitoring water conditions in polluted areas (Volpe et al. 2020). Research by Bergey and Weis (2007), Protasowicki et al. (2013), and Mackevičiené (2002) showed that in the case of numerous aquatic crustaceans, i.e. shrimps, crabs, or crayfish, toxic metals tend to accumulate in the exoskeleton. This tissue, therefore, has a very important detoxification function because toxic substances are removed from the animal’s body during the moult. The main component of the shells of crustaceans is chitin (poly-β-(1,4)-N-acetyl-D-glucosamine), a natural biopolymer characterised by high bioactivity, biodegradability, and non-toxicity, as well as good chelating property of metal ions (Bhatnagar and Sillanpää 2009; Jaafarzadeh et al. 2015; de Sousa et al. 2020; Złotko et al. 2021). Therefore, chitosan obtained from chitin is already widely used in medicine in nerve or wound repair and in agriculture as a pesticide, growth stimulator, and fruit preservative, but also in environmental protection in the treatment of wastewater (Zhang et al. 2022). Could the spiny-cheek exoskeleton be a rich source of divalent metals such as Zn, Cu, and Ca?
The degree of accumulation of metals in tissues is influenced by physical and chemical factors, i.e. pH, salinity, and temperature of the environment, exposure time, metal concentration (Anastopoulos et al. 2017; Jaafarzadeh et al. 2015), and biological ones (i.e. tissue type, species, size, body condition, and eating habits) (Balzani et al. 2022; Evans-Illidge 1997; Zhang et al. 2019). The accumulation level of metals in crayfish tissues is a good indicator of contamination of the aquatic environmental (Goretti et al. 2016; Kuklina et al. 2014; Varol and Sünbül 2018). Crayfish can accumulate metals from sediment (Varol and Sünbül 2018), water, or food (Mistri et al. 2020). The research of Keteles and Fleeger (2001) confirms that the tendency to accumulate metals in the exoskeleton of crustaceans is an individual property of the organism and is highly dependent on the type of metal. Therefore, an analysis of age and sex of spiny-cheek crayfish was undertaken.
North American spiny-cheek crayfish (Faxonius limosus [Rafinesque 1817]) has been widely introduced in Poland, disrupting the local biodiversity (Krzywosz 2004; Holdich and Black 2007). Due to its tolerance of a wide range of environmental conditions, including chemically contaminated and eutrophicated reservoirs (Buřič et al. 2013) and its immunity to crayfish plague due to the new genotype of pathogen Aphanomyces astaci, it poses a threat to native noble crayfish (Astacus astacus [Linnaeus 1758]) and mud crayfish (Astacus leptodactylus [Eschscholz 1823]) (Śmietana 2000). In accordance with the Regulation of the Minister of Agriculture and Rural Development of November 12, 2001 (Journal of Laws No. 138, item 1559) with the amendment introduced in paragraph 8 (Journal of Laws of 2003, No. 17, item 160), spiny-cheek crayfish should be eradicated and the release of captured individuals is not allowed.
The aim of the study was to analyse the effect of age and sex of crayfish on the degree of accumulation of selected metals (Ca, Cu, and Zn) in the muscle and exoskeleton, to analyse correlations between the metal concentrations in different tissues and the total length of crayfish, to analyse the bioaccumulation coefficient, and to assess the percentage of the analysed metals in the water, sediment, muscle and exoskeleton. Such complex analyses and interactions can improve our understanding of metal absorption, as well as enable more accurate estimates of the amount of bioavailable metals present at a given site. Additionally, the determined concentrations of elements with recommendations for their daily intake were compared.
The research hypothesis states that the age of crayfish, to a greater extent than sex, influences the degree of metal accumulation in crayfish tissues. As spiny-cheek crayfish are benthic crustaceans, the BCF of metals may be higher from sediment than from water.
Material and methods
Study site and sampling
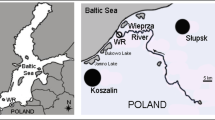
A total of 50 males and 50 females of the spiny-cheek crayfish were caught in autumn (October 2019) using fyke nets. The traps were placed in the coastal zone of the Głowińsk reservoir near Rypin (north-central Poland) (Fig. 1). Only healthy individuals were included in the study, and crayfish with damaged claws were not considered for the analyses. After washing, the crayfish were placed in water and transported to the laboratory.
The age of the crayfish was determined following the size-age classification of Pieplow (1938). All males occurred as form I (reproductively active form with a well-developed first pair of pleopods) according to the description of Chybowski (2007) and Pielplow (1938). Sexually mature individuals of total length from 80.7 to 104.2 mm (measured from the tip of the rostrum to the end of the telson) have been collected (Table 1). Spiny-cheek crayfish in Polish climatic conditions become sexually mature in the first or second year of life (Holdich and Black 2007; Chybowski 2007) when they reach a total length of 60 mm (Pielplow 1938; Juchno and Chybowski 2003).
Water samples were taken from the subsurface layer using water sampling pole from four sampling points (ISO 5667–4:2016). One litre of average sample (1000 ml) was filtered using WhatmanTM grade 1 qualitative filter paper and collected in a polyethylene (PE) container. The sample was acidified to pH 1 − 2 with concentrated nitric acid (ISO 5667–3:2018). The bottom sediment samples were collected from the same four sampling points using an Ekman-Grab sampler of 2250 ml volume. One litre of average sample (1000 ml) was collected in a polyethylene (PE) container and stored in the refrigerator for analysis (ISO 5667–12:2017). Directly after sampling and transportation, the sample was freeze dried (lyophilized) and stored at − 20 °C.
Metal sample preparation and analysis
For analysis, the abdominal muscle and carapace part of exoskeleton of individuals during inter-moult stage were collected. Crayfish samples of abdominal muscle were preserved in the freezer at − 20 ℃ until further processing. Samples of exoskeleton were air dried. All samples were then freeze-dried in Lyovac GT2 freeze drier by Finn-Aqua (Finland) (parameters: temperature: – 40 °C, pressure: 6·10−2 mbar, and duration at least 48 h).
Cu, Zn, and Ca concentrations were determined in freeze-dried samples after aqua regia digestion (ISO 11466:1995) on Thermo Scientific iCE 3000 SERIES spectrophotometer, calibrated using Merck standard solutions (Merck KGaA). The validation of the analysis was conducted on certified standards—certified reference material ERM®-BB422 fish muscle, certified reference material BCR®-670 aquatic plant, and certified reference material CRM027-050 sandy loam 10. The limit of detection (LOD) and the limit of quantification (LOQ) for each element was calculated from the standard deviation of blank samples (S) as LOD = 3 S and LOQ = 10 S (Shehata et al. 2018) (Table 2). The metals in water were determined directly after sampling and transportation. The concentrations of the metals were calculated from linear calibration plots obtained by measurement of the standard solutions. All determinations were made in triplicate and the data for samples of the muscle, exoskeleton and bottom sediments were corrected to oven-dry (105 °C) moisture content (given in mg kg−1 dry weight—mg kg−1 dw for Cu and Zn and g kg−1 dry weight—g kg−1 dw for Ca).
Bioconcentration factor
The accumulation of metals in crayfish tissue was measured using BCF. According to Jitar et al. (2015) and Vrhovnik et al. (2013), BCF is defined as follows:
where Cb is the concentration of metals in the muscle/exoskeleton; C is the concentration of metals in sediments/water.
Statistical analyses
Statistical calculations were made using Statistica 13.0 software (StatSoft 13.0). Significant differences between the groups were tested with a two-way analysis of variance (ANOVA), and Tukey’s test was used for multiple comparisons. The normality of the data was tested using the Shapiro–Wilk’s test, and the homogeneity of variance was verified by means of the Levene’s test. The level of significance was set at P ≤ 0.05. Linear regression analysis was employed to identify relationships between metal concentrations and total length (TL) of spiny-cheek crayfish in the individual tissues for each age and sex group. To assess how well a statistical model predicted an outcome, the coefficient of determination (R2) was also taken into account, which determines the proportion of variance in the dependent variable that can be explained by the independent variable.
Results
Our analyses indicated that metals accumulated in the following sequence: Ca > Zn > Cu in the abdominal muscle, exoskeleton, water, and bottom sediment (Table 3). Figure 2 shows the results of two-way ANOVA for each metal and tissue using both age and sex as predictors, including the values of F and P. Sex was a significant factor affecting the level of Ca in the muscle and Zn in the exoskeleton. The age of the crayfish was a significant factor affecting the level of Zn, Cu, and Ca in the muscle and Zn and Cu in the exoskeleton, mainly in the male group. The effect of interaction between the analysed variables (age:sex) in the case of Zn and Ca for the muscle and in the case of Cu for the exoskeleton was confirmed (this is shown by the crossed lines on the graphs). For example, the effect of one factor (age) on the dependent variable (Zn) in the muscle changes depending on the effect of the other factor (sex). Significantly high levels of Zn (16.06 mg kg−1 dw) and Cu (3.96 mg kg−1 dw) were determined in the exoskeleton of 3-year-old individuals. In the case of muscle, significantly high values were found for Zn (117.42 mg kg−1 dw) and Ca (5.91 g kg−1 dw) in 4-year-old individuals, while the concentration of Cu was nearly 7 mg kg−1 dw higher in the tissue of 3-year-old crayfish compared with 4-year-old ones.
The highest amounts of Zn were found in the muscle of 4-year-old females, Cu was determined in the highest amounts in 3-year-old males, and the level of Ca was significantly high in the muscle of 4-year-old males (Table 4). Significant differences were found in the concentration of Zn between 3- and 4-year-old females and in the concentration of Cu between 3- and 4-year-old males. Considerably high values of Zn and Cu were found in the exoskeleton of 3-year-old males. A significantly low level of Zn was determined in the tissue of 4-year-old males. Ca concentrations ranged from 157.18 g kg−1 dw (3-year-old males) to 171.50 g kg−1 dw (4-year-old females), but these values were not significantly different between groups (Table 4).
Figure 3 shows the percentage of the analysed metals in water, sediment, muscle, and exoskeleton. Zn and Cu accumulated in the greatest amounts in the muscle (from 62.60 to 77.17%), and in the smallest amounts in the exoskeleton (from 4.87 to 12.30%). Ca was most abundant in the exoskeleton (77.30% in 4-year-old females to 75.13% in 4-year-old males). In the muscle, the percentage of Ca ranged from 1.04% in 4-year-old females to 3.58% in 4-year-old males).
The ability of an aquatic organism to absorb chemicals from the environment can be assessed by the BCF. Table 5 shows the values of BCF for each group of individuals. The accumulation coefficient was higher in the muscle than in the exoskeleton for Zn and Cu, and vice versa for Ca. BCF of Zn and Cu in the muscle and exoskeleton of spiny-cheek crayfish was much higher from water than from sediments, unlike Ca.
Results of linear regression analysis with metal concentration as dependent variable and total length (TL) as independent variable (predictor) were presented in Table 6 (for muscle) and in Table 7 (for exoskeleton). Only in a few cases did the concentration of metals in the muscle significantly correlate with the total length (TL) of the crayfish. A statistically significant correlation (P < 0.05) between Zn concentration and TL in 3-year-old females and 4-year-old males and between Cu concentration and TL in 3-year-old males was observed (Table 6). The analyses of the coefficient of determination (R2), which informs us about the proportion of the variance for a dependent variable that is explained by an independent variable in a regression model, confirmed that the introduced predictor account for 55% to almost 90% of the total variation in its outcome variable (R2 = 0.5484, R2 = 0.7661, and R2 = 0.8989).
Table 8 presents the content of Ca, Cu, and Zn determined in the muscle of crayfish calculated for 100 g of wet weight and % of the RDI for the analysed minerals. The research indicated that crayfish muscle was a rich source of Cu (over 100% RDI), and consumption of 100 g of spiny-cheek crayfish muscle could meet the daily requirement for Zn up to 27.5% and for Ca in 12.4%.
Discussion
Supporting previous literature, Ca accumulated in the highest amounts in the exoskeleton, while Zn and Cu in the muscle (Nędzarek et al. 2020; Śmietana et al. 2020). Our research confirmed that concentration of metals in the abdominal muscle and the exoskeleton of spiny-cheek crayfish was in this decreasing order Ca > Zn > Cu. The same results for muscle were observed by Protasowicki et al. (2013) in signal crayfish (Pacifastacus leniusculus [Dana 1852]) from Mazurian Lakes, by Goretti et al. (2016) in red swamp crayfish (Procambarus clarkii [Girard 1852]) from Lake Trasimeno, and by Varol and Sünbül (2018) in mud crayfish (Astacus leptodactylus [Eschscholz 1823]) from the Keban Dam Reservoir in Turkey. A higher level of Cu than Zn in exoskeleton was observed by Nędzarek et al. (2020) in signal crayfish exoskeleton from the Wieprza River and by Mistri et al. (2020) in red swamp crayfish of the Po River Delta area.
Calcium (Ca) is one of the macroelements responsible for building the skeletal system and maintaining a proper acid–base balance, and as confirmed by the research of Lall (2002), muscle tissue is not the primary site of Ca accumulation, unlike the scales, bones, and skin. This was confirmed by our analyses of a spiny-cheek crayfish, which showed calcium concentrations about 40 times higher in the exoskeleton compared to the muscle tissue (Table 3). This is due to chitin, which builds the crustacean exoskeleton, which is made of calcium carbonate and has a high sorption capacity for metals, which is due to passive adsorption of metals from water (Mistri et al. 2020). Hence, this tissue may be a rich source of Ca and can be used as a natural fertiliser for plants. Nędzarek et al. (2020) recorded 260 to 500 times higher Ca level in the exoskeleton compared to the muscle for the signal crayfish. Our research did not confirm significant differences in the level of Ca in the exoskeleton between groups. Neither sex nor age was a significant factor affecting the level of Ca in this tissue. As Wærvågen et al. (2016) confirmed, moult timing and frequency, as well as length increment per moult, have a major impact on exoskeleton calcification in Norwegian populations of the noble crayfish.
Zn is a microelement that plays an important role in the proper functioning of an organism. This metal is responsible for carbohydrates, proteins, nucleic acid metabolism, proper bone mineralization, and the functioning of the immune system (Chavez-Sanchez et al. 2000). Our analyses of spiny-cheek crayfish demonstrated about 8.5 times higher concentration of Zn in muscle compared to the exoskeleton (Table 3). Nędzarek et al. (2020) found about 3 times higher Zn levels in signal crayfish muscle compared to the exoskeleton. The same results were denoted by Mistri et al. (2020) in red swamp crayfish (the ratio was from 3.5 to 4.8 times). The studies of Jaafarzadeh et al. (2015) confirmed that the chitin extracted from the shrimp was an effective Zn adsorbent, but the contact time or individual predispositions strongly influenced the efficiency of biosorption.
A microelement that plays a significant role in the production of red blood cells, synthesis of nucleic acids, hardening of collagen and keratinization of hair, haemocyanin biosynthesis, and catabolism in aquatic arthropods and molluscs is copper (Cu) (Mistri et al. 2020). Our research showed almost 9 times higher concentration of this metal in muscle compared to the exoskeleton (Table 3). The highest concentration of this metal in the exoskeleton was found in 3-year-old males of spiny-cheek crayfish. Nędzarek et al. (2020) found about 1.3 times higher Cu levels in signal crayfish muscle compared to the exoskeleton. The same results were denoted by Mistri et al. (2020) in red swamp crayfish (the ratio was from 1.3 to 3.4 times). The studies of Soedarini et al. (2012) on red swamp crayfish showed that the muscle tissue was characterised by the lowest degree of Cu absorption compared to other tissues, which confirms a greater degree of elimination than accumulation. No significant differences in Cu concentration in the abdominal muscle between females and males of spiny-cheek crayfish within one age group were observed. The only significant differences were found in the Cu concentration in muscle between 3- and 4-year-old males, which were in line with Balzani et al. (2022). These differences are likely due to the ability of crayfish to clear metals rapidly, and this is why these animals are useful for assessing Cu bioavailability in aquatic ecosystems in a short-term monitoring programme (Kouba et al. 2010; Xiong et al. 2020). As demonstrated by Mistri et al. (2020), the large accumulation of Cu in the abdominal muscle may also be caused by a physiological response to external stress.
Statistically significant differences in the content of the determined metals between 3- and 4-year-old crayfish were determined for Zn, Cu and Ca in the muscle and for Cu and Zn in the exoskeleton. The analyses showed significant differences in the concentration of Zn in muscle between 3- and 4-year-old females and in the concentration of Cu and Ca between 3- and 4-year-old males. As confirmed by Jaafarzadeh et al. (2015) in studies performed on shrimp shells, the period of exposure to the environmental factor is responsible for the level of accumulation of metals such as Zn. This is consistent with our previous research, which confirmed the significant influence of the age of spiny-cheek crayfish on the Zn and Cu levels in muscle (Stanek et al. 2017). Lower metal values in the muscle of older individuals (for example, Cu in 4-year-old spiny-cheek crayfish) may be due to a dilution effect (Balzani et al. 2022). When the metabolic rate is faster, as is often the case in younger organisms, then the growth dilution effect will be greater (Evans-Illidge 1997).
Statistically significant differences in the content of the determined metals between males and females were determined for Ca in the muscle and for Zn in the exoskeleton. Hagen and Sneddon (2009) and Nędzarek et al. (2020) showed no significant difference between males and females crayfish in Zn and Cu content in the muscle. Naghshbandi et al. (2007) confirmed differences in Zn and Cu content in the muscle between sexes for mud crayfish. Chen et al. (2005) confirmed the lack of significant differences in Cu concentration between individuals of different sexes in any tissue. One hypothesis is that Cu is an essential element for the blood pigments of crustaceans, and its requirement should not differ between the sexes. Differences in the concentration of this metal may appear in small crustaceans or juveniles with little or no haemocyanin, which may result in an increase in the concentration of Cu in the body as the size of the animal increases. Kaya et al. (2015) confirmed by scanning electron microscopy (SEM) that the male chitin surface structure contained 25–90 nm wide nanofibres and 90–250 nm nanopores, while no pores or nanofibres were observed in the chitin surface structure of the majority of females (nanofibres were observed only in Melanogryllus desertus females). Moreover, they confirmed the differences in dry matter of chitin between species (these values ranged from 4.71 to 11.84%), and they observed that the quantity of chitin was greater in males than in females. The research of Jussila et al. (1995) on noble crayfish confirmed differences in the metal content of the exoskeleton between males and females may be due to the difference in the frequency of moulting during the year. This was confirmed by the research of Nędzarek et al. (2020) on the level of metals in the exoskeleton of signal crayfish from the Wieprza River. Differences in the metal content of the muscle between males and females may be due to differing growth rates between sexes (Evans-Illidge 1997). Moreover, research concerning Nephrops norvegicus shows that males have higher feeding rates than females, and the concentration of metals may be higher (Canli and Furness 1993).
According to Tao et al. (2012), a BCF value > 1 means that the organism has the potential to accumulate a chemical substance, and BCF value > 100 means high accumulation capacity. Other literature sources indicate that a BCF value of < 1000 means that the chemical is not significantly bioaccumulative, with BCF in the range of 1000–5000, the chemical substance has a potential to bioaccumulate, and with BCF > 5000, the chemical substance shows high bioaccumulation (Costanza et al. 2012; Varol and Sünbül 2018). The calculated BCF values for Zn were consistent with the data obtained by Nędzarek et al. (2020) for signal crayfish, and BCF values for Cu were the same as those obtained by Varol and Sünbül (2018) for mud crayfish. Our analyses confirmed that Zn and Cu accumulate mainly in the muscle and Ca in the exoskeleton. This is because Ca is the main constituent of the exoskeleton as calcium carbonate (30–40%), in addition to chitin (20–30%) and protein (20–30%) (Chen et al. 2020). The analyses of spiny-cheek crayfish showed a much higher bioaccumulation capacity of Zn and Cu in the muscle from water (BCF > 1000) and a greater bioaccumulation capacity of Ca in the exoskeleton from sediments (BCF > 3). Since the exoskeleton is in direct contact with the environment, metals accumulate in this tissue through passive adsorption from water or sediment rather than bioaccumulation (Mistri et al. 2020). The differences in the bioaccumulation of metals in tissue by elimination compared to other tissues can be expressed as the ratio of the levels found in these tissues (for example, ratio exoskeleton/muscle). BCF values for Zn and Cu were similar and were below 1, while in the case of Ca, BCF value was in the range of 20.98 to 74.24. Two factors influence the BCF values in different tissues: different biochemical functions of elements and different physiological functions of individual organs (Nędzarek et al. 2020). In order to confirm this tendency of metal accumulation in the analysed crayfish species, studies on individuals obtained from reservoirs with various degrees of eutrophication should be carried out.
The lack of significant relationships between total length and the concentration of metals in the muscle may result from the dilution of metals as the animal's body grows, moreover, it may indicate faster metabolism in younger animals compared to older ones (Balzani et al. 2022), and it may also result from the growth-hindering effect of metals. Conversely, a positive correlation indicates a trend of metal accumulation as the animal grows (Ergen et al. 2015). Significant negative correlations between the concentration of metals and TL in the exoskeleton may result from cyclic moulting, which results in the removal of metals. Our analyses on spiny-cheek crayfish supported this. The degree of Zn accumulation in crayfish tissues depends on the concentration of Ca in the water and the correlation between these metals, and it is largely due to surface adsorption on the shell and gills (Bryan 1967). The hypothesis is that Ca has the propensity to form complexes with phytate and Zn that are insoluble and consequently have an inhibitory effect on Zn absorption (Lönnerdal 2000). Our preliminary analyses confirmed the lack of significant correlations between the concentration of Zn and Ca in each of the studied groups.
The results of the RDI analysis for spiny-cheek crayfish were in line with Nędzarek et al. (2020), who showed that the consumption of a portion of 100 g of signal crayfish muscle from the Wieprza River meets 3 to 10% of the dietary reference intake (DRI) for K, Ca, and Fe. In the case of Zn DRI, it was lower for muscle from abdomen of males and females (about 23%) and much higher for muscle with their claws (over 60%). Only in the case of Cu was DRI exceeded, with the range from 88 to 164% of DRI. As confirmed by Śmietana et al. (2021), the consumption of 100 g of chelae muscle of spiny-cheek crayfish from Lake Sominko covers 103.7 and 54.8% of the consumer’s daily demand for Zn and Ca, respectively. The consumption of 100 g of abdomen muscle mostly covers the demand for Zn (17.08%). Moreover, according to the data of the US Food and Drugs Administration, the maximum allowable level is 6.1 mg kg−1 dw for Cu and 21.2 mg kg−1 dw for Zn (Alcorlo et al. 2006), which makes the analysed spiny-cheek crayfish suitable for human consumption.
Conclusions
Factors influencing the differences in the accumulation of metals in crayfish tissues are age, which is equivalent to the time of exposure of the animal to metals, and sex, which determines the moulting cycle, different physiological conditions, and elimination processes. This hypothesis was supported by spiny-cheek crayfish analyses. For most metals, significant differences were found between 3- and 4-year-old individuals both in the muscle and exoskeleton. The analyses of spiny-cheek crayfish partially supported the hypothesis of the BCF, as this mechanism depends on the type of metal. Knowledge of the mechanisms of metal bioaccumulation and the factors influencing it is an important contribution to the environmental monitoring of freshwater ecosystems. As evidenced by the studies of other authors, environmental conditions are very important, especially pH (which affects the degree of mineralization); therefore, this mechanism requires further research. Analyses of the RDI for the investigated minerals and data from the US Food and Drugs Administration, the maximum allowable level of Zn and Cu confirmed that consumption of crayfish meat would be safe for the health of potential consumers in terms of Ca, Zn, and Cu levels.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Materials availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Alcorlo P, Otero M, Crehuet M, Baltanás A, Montes C (2006) The use of the red swamp crayfish (Procambarus clarkii, Girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci Total Environ 366(1):380–390. https://doi.org/10.1016/j.scitotenv.2006.02.023
Anastopoulos I, Bhatnagar A, Bikiaris D, Kyzas G (2017) Chitin adsorbents for toxic metals: a review. Int J Mol Sci 18(1):114. https://doi.org/10.3390/ijms18010114
Balzani P, Haubrock PJ, Russo F, Kouba A, Haase P, Veselý L, Masoni A, Tricarico E (2021) Combining metal and stable isotope analyses to disentangle contaminant transfer in a freshwater community dominated by alien species. Environ Pollut 115781. doi:https://doi.org/10.1016/j.envpol.2020.115781
Balzani P, Kouba A, Tricarico E et al (2022) Metal accumulation in relation to size and body condition in an all-alien species community. Environ Sci Pollut Res 29:25848–25857. https://doi.org/10.1007/s11356-021-17621-0
Barrento S, Marques A, Teixeira B, Analceto P, Carvalho ML, Vaz-Pires P, Nunes ML (2009) Macro and trace elements in two populations of brown crab Cancer pagurus: ecological and human health implications. J Food Compos Anal 22:65–71. https://doi.org/10.1016/j.jfca.2008.07.010
Bergey LL, Weis JS (2007) Molting as a mechanism of depuration of metals in the fiddler crab. Uca Pugnax Mar Environ Res 64(5):556–562. https://doi.org/10.1016/j.marenvres2007.04.009
Bhatnagar A, Sillanpää M (2009) Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater — a short review. Adv Colloid Interface Sci 152(1–2):26–38. https://doi.org/10.1016/j.cis.2009.09.003
Bo L (2000) Dietary factors influencing zinc absorption. J Nutr 130(5):1378S-1383S. https://doi.org/10.1093/jn/130.5.1378S
Bryan GW (1967) Zinc regulation in the freshwater crayfish (including some comparative copper analyses). J Exp Biol 46:281–296
Buřič M, Kouba A, Kozák P (2013) Reproductive plasticity in freshwater invader: from long-term sperm storage to parthenogenesis. PLoS ONE 8(10):e77597. https://doi.org/10.1371/journal.pone.0077597
Canli M, Furness RW (1993) Heavy metals in tissues of the Norway lobster Nephrops norvegicus: effects of sex, size and season. Chem Ecol 8(1):19–32. https://doi.org/10.1080/02757549308035297
Chavez-Sanchez C, Martinez-Palacios CA, Martinez-Perez G, Ross LG (2000) Phosphorus and calcium requirements in the diet of the American cichlid Cichlasoma urophthalmus (Günther). Aquac Nutr 6:1–9. https://doi.org/10.1046/j.1365-2095.2000.00118.x
Chen M-H, Chen C-Y, Chou H-Y, Wen T-C (2005) Gender and size effects of metal bioaccumulation on the rock crab, Thalamita crenata, in Dapeng Bay, Southwestern Taiwan. Mar Pollut Bull 50:463–484. https://doi.org/10.1016/j.marpolbul.2005.01.012
Chen J, Jiang S, Jiang H (2020) A review on conversion of crayfish-shell derivatives to functional materials and their environmental applications. J Bioresour Bioprod 5(4):238–247. https://doi.org/10.1016/j.jobab.2020.10.002
Chybowski Ł (2007) Morphometric, fecundity, density, and feeding intensity of the spiny-cheek crayfish, Orconectes limosus (Raf.) in natural condition. Arch Pol Fisch 15(3):175–241. https://www.infish.com.pl/wydawnictwo/Archives/Fasc/Vol15_Fasc3.html
Costanza J, Lynch DG, Boethling RS, Arnot JA (2012) Use of the bioaccumulation factor to screen chemicals for bioaccumulation potential. Environ Toxicol Chem 31(10):2261–2268. https://doi.org/10.1002/etc.1944
Ergen FŞ, Tunca EÜ, Ozkan AD, Ölmez TT, Acaröz E, Altındağ A, Tekinay T, Tunca E (2015) Interactions between metals accumulated in the narrow-clawed crayfish Astacus leptodactylus (Eschscholtz, 1823) in Dikilitaş Lake. Turkey Chem Ecol 31(5):455–465. https://doi.org/10.1080/02757540.2015.1050002
Evans-Illidge E (1997) Heavy metals in commercial prawn and crayfish species in Torres Strait Great Barrier Reef Marine Park Authority ISBN 0 642 26442 2. https://elibrary.gbrmpa.gov.au
Goretti E, Pallottini M, Ricciarini MI, Selvaggi R, Cappelletti D (2016) Heavy metals bioaccumulation in selected tissues of red swamp crayfish: an easy tool for monitoring environmental contamination levels. Sci Total Environ 559:339–346. https://doi.org/10.1016/j.scitotenv.2016.03.169
Hagen JP, Sneddon J (2009) Determination of copper, iron, and zinc in crayfish (Procambrus clarkii) by inductively coupled plasma–optical emission spectrometry. Spectrosc Lett 42(1):58–61. https://doi.org/10.1080/00387010802375065
Heidarieh M, Maragheh MG, Shamami MA et al. (2013) Evaluate of heavy metal concentration in shrimp (Penaeus semisulcatus) and crab (Portunus pelagicus) with INAA method. SpringerPlus 2–72. https://doi.org/10.1186/2193-1801-2-72
Holdich D, Black J (2007) The spiny-cheek crayfish, Orconectes limosus (Rafinesque, 1817) [Crustacea: Decapoda: Cambaridae], digs into the UK. Aquat Invasions 2(1):1–15. https://doi.org/10.3391/ai.2007.2.1.1
ISO 5667–3:2018: Water quality - sampling - part 3: preservation and handling of water samples
ISO 5667–4:2016: Water quality - sampling - part 4: guidance on sampling from lakes, natural and man-made
ISO 5667–12:2017: Water quality - sampling - part 12: guidance on sampling of bottom sediments from rivers, lakes and estuarine areas
Jaafarzadeh N, Mengelizadeh N, Takdastan A, Farsani MH, Niknam N, Aalipour M et al (2015) Biosorption of heavy metals from aqueous solutions onto chitin. Int J Env Health Eng 4:1–7. https://doi.org/10.4103/2277-9183.153992
Jarosz M, Rychlik E, Stoś K, Charzewska J (2020) Standards of nutrition for the Polish population - amendment. Publisher: Institute of Food and Nutrition in Warsaw (In Polish)
Jitar O, Teodosiu C, Oros A, Plavan G, Nicoara M (2015) Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. N Biotechnol 32:369–378. https://doi.org/10.1016/j.nbt.2014.11.004
Juchno D, Chybowski Ł (2003) Histological analyses of gonad development in female spiny-cheek crayfish (Orconectes limosus RAF). Arch Pol Fish 11(1):69–78
Jussila J, Henttonen P, Huner JV (1995) Calcium, magnesium, and manganese content of noble crayfish (Astacus astacus (L.) branchial carapace and its relationship to water and sediment mineral contents of two ponds and one lake in central Finland, Freshwater Crayfish 10:230–238. https://www.researchgate.net/publication/233341441Accessed 28 May 2014
Kaya M, Lelešius E, Nagrockaitė R, Sargin I, Arslan G, Mol A et al (2015) Differentiations of chitin content and surface morphologies of chitins extracted from male and female grasshopper species. PLoS ONE 10(1):e0115531. https://doi.org/10.1371/journal.pone.0115531
Keteles KA, Fleeger JW (2001) The contribution of ecdysis to the fate of copper, zinc and cadmium in grass shrimp Palaemonetes pugio Holthius. Mar Pollut Bull 42(12):1397–1402. https://doi.org/10.1016/S0025-326X(01)00172-2
Kouba A, Buřič M, Kozák P (2010) Bioaccumulation and effects of heavy metals in crayfish: a review. Water Air Soil Pollut 211(1–4):5–16. https://doi.org/10.1007/s11270-009-0273-8
Krzywosz T (2004) Is it reverse of spiny cheek crayfish? Komun Ryb 5:21–23
Kuklina I, Koub A, Buřič M, Horká I, Ďuri Z, Kozák P (2014) Accumulation of heavy metals in crayfish and fish from selected Czech reservoirs. BioMed Res Int 306103:1–9. https://doi.org/10.1155/2014/306103
Lall SP (2002) The minerals. In: Halver J.E. and Hardy R.W, Fish Nutrition pp 259–308
Mackevičiené G (2002) Bioaccumulation of heavy metals in noble crayfish (Astacus astacus L.) tissues under aquaculture conditions. Ekol (vilnius) 2:79–82
Mistri M, Munari C, Pagnoni A, Chenet T, Pasti L, Cavazzini A (2020) Accumulation of trace metals in crayfish tissues: is Procambarus clarkii a vector of pollutants in Po Delta inland waters? Eur Zool J 87(1):46–57. https://doi.org/10.1080/24750263.2020.1717653
Naghshbandi N, Zare S, Heidari R, Razzaghzadeh S (2007) Concentration of heavy metals in different tissues of Astacus leptodactylus from Aras Dam of Iran. Pak J Biol Sci 10(21):3956–3959. https://doi.org/10.3923/pjbs.2007.3956.3959
Nędzarek A, Czerniejewski P, Tórz A (2020) Macroelements and trace elements in invasive signal crayfish (Pacifastacus leniusculus) from the Wieprza River (Southern Baltic): human health implications. Biol Trace Elem Res 197:304–315. https://doi.org/10.1007/s12011-019-01978-y
Pielplow U (1938) Fischerieiwissenschaftliche Monographie von Cambarus affinis Say. Z Fischerei Hilfswiss 36(16):350–437
Protasowicki M, Własow T, Rajkowska M, Polna M, Bernad A (2013) Metal concentrations in selected organs of crayfish—Orconectes limosus and Pacifastacus leniusculus from Mazurian Lakes. J Elementol 1:683–694. https://doi.org/10.5601/jelem.2013.18.4.537
Raissy M, Ansari M, Rahimi E (2011) Mercury, arsenic, cadmium and lead in lobster (Panulirus homarus) from the Persian Gulf. Toxicol Ind Health 27(7):655–659. https://doi.org/10.1177/0748233710395346
Regulation of the Minister of Agriculture and Rural Development of November 12, 2001 (Journal of Laws No. 138, item 1559) (In Polish)
Shehata AB, Yamani RN, Tahoun IF (2018) Validation of a method for quantification of lead, chromium, magnesium, zinc & copper in human blood and serum using atomic absorption spectrometry. J Phys: Conf Ser 1065:242002. https://doi.org/10.1088/1742-6596/1065/24/242002
de Sousa VR, da Cunha Santos MA, de Sousa VB, de Araújo NG, Navarro de Lima SL, Menezes RR (2020) A review on chitosan’s uses as biomaterial: tissue engineering, drug delivery systems and cancer treatment. Materials (basel) 3(21):4995. https://doi.org/10.3390/ma13214995
Soedarini B, Klaver L, Roessink I, Widianarko B, van Straalen NM, van Gestel CAM (2012) Copper kinetics and internal distribution in the marbled crayfish (Procambarus sp.). Chemosphere 87(4):333–338. https://doi.org/10.1016/j.chemosphere.2011.12.017
Stanek M, Dąbrowski J, Sz R, Janicki B, Długosz J (2017) Heavy metals bioaccumulation in tissues of spiny-cheek crayfish (Orconectes limosus) from Lake Gopło: effect of age and sex. Bull Environ Contam Toxicol 98(6):740–746. https://doi.org/10.1007/s00128-017-2098-2
Śmietana P (2000) The problem of active protection of native crayfish in Poland. Mag Przem Ryb 1(13):22–23
Śmietana N, Panicz R, Sobczak M, Nędzarek A, Śmietana P (2020) Variability of elements and nutritional value of spiny-cheek crayfish (Faxonius limosus, Rafinesque, 1817). J Food Comp Anal 94(103656). DOI:https://doi.org/10.1016/j.jfca.2020.103656
Śmietana N, Panicz R, Sobczak M, Śmietana P, Nędzarek A (2021) Spiny-cheek crayfish, Faxonius limosus (Rafinesque, 1817), as an alternative food source. Animals 11(1):59. https://doi.org/10.3390/ani11010059
Tao Y, Yuan Z, Xiaona H, Wein M (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotox Environ Safe 81:55–64. https://doi.org/10.1016/j.ecoenv.2012.04.014
Wærvågen SB, Andersen T, Trond T (2016) Exoskeleton calcification in Norwegian populations of the crayfish Astacus astacus (Linnaeus, 1758) (Decapoda: Astacidae) varies with size, gender, and ambient calcium concentration. J Crustac Biol 36(2):189–197. https://doi.org/10.1163/1937240x-00002406
Varol M, Sünbül MR (2018) Biomonitoring of trace metals in the Keban Dam Reservoir (Turkey) using mussels (Unio elongatulus eucirrus) and crayfish (Astacus leptodactylus). Biol Trace Elem Res 185(1):216–224. https://doi.org/10.1007/s12011-017-1238-1
Volpe MG, Ghia D, Safari O et al (2020) Fast non-destructive assessment of heavy metal presence by ATR–FTIR analysis of crayfish exoskeleton. Environ Sci Pollut Res 27:21021–21031. https://doi.org/10.1007/s11356-020-08405-z
Vrhovnik P, Arrebola JP, Serafimovski T, Dolenec T, Smuc NR, Dolenec M, Mutch E (2013) Potentially toxic contamination of sediments, water and two animal species in Lake Kalimanci, FYR Macedonia: relevance to human health. Environ Pollut 180:92–100. https://doi.org/10.1016/j.envpol.2013.05.004
Xiong B, Xu T, Li R-p et al (2020) Heavy metal accumulation and health risk assessment of crayfish collected from cultivated and uncultivated ponds in the Middle Reach of Yangtze River. Sci Total Environ 736: https://doi.org/10.1016/j.scitotenv.2020.139963
Złotko K, Waśko A, Kamiński DM, Budziak-Wieczorek I, Bulak P, Bieganowski A (2021) Isolation of chitin from black soldier fly (Hermetia illucens) and its usage to metal sorption. Polymers 13(5):818. https://doi.org/10.3390/polym13050818
Zhang Z, Fang Z, Li J, Sui T, Lin L, Xu X (2019) Copper, zinc, manganese, cadmium and chromium in crabs from the mangrove wetlands in Qi’ao Island, South China: levels, bioaccumulation and dietary exposure. Watershed Ecol Environ 1:26–32. https://doi.org/10.1016/j.wsee.2019.09.001
Zhang M, Zhang F, Li C, An H, Wan T, Zhang P (2022) Application of chitosan and its derivative polymers in clinical medicine and agriculture. Polymers (Basel) 28;14(5):958. DOI: https://doi.org/10.3390/polym14050958.
Funding
The study was supported by departmental sources only, and grants were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection were performed by Bogusław Chachaj and Magdalena Stanek. Analysis was performed by Szymon Różański. The first draft of the manuscript was written by Magdalena Stanek.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. All efforts were made to minimize suffering according to Polish law and the EU Directive (no. 2010/63/EU). All members of the research team were trained in animal care, handling, and euthanasia by PolLASA (Polish Laboratory Animal Science Association).
Consent to participate
All authors confirmed their participation in the publication.
Consent for publication
All authors read and approved the final manuscript and approved the version to be published.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stanek, M., Chachaj, B. & Różański, S. Factors influencing accumulation of Zn, Cu, and Ca in the tissues of spiny-cheek crayfish (Faxonius limosus, Rafinesque, 1817). Environ Sci Pollut Res 30, 44161–44172 (2023). https://doi.org/10.1007/s11356-023-25318-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25318-9