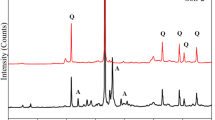
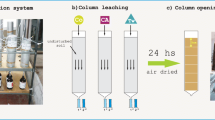
Abstract
Understanding the mobility, retention, and fate of carbon dots (CDs) is critical for the risk management of this emerging carbon material. However, the influences of surfactants on CDs’ transport through subsurface media are still poorly understood. Herein, column experiments were conducted to explore the different influences of an anionic surfactant, sodium dodecylbenzene sulfonate (SDBS), and a cationic surfactant, cetyltrimethylammonium bromide (CTAB), on the CDs’ transport in water-saturated soil. In the Na+ background electrolyte, both surfactants facilitated the transport of CDs at pH 7.0. The trend stemmed from steric hindrance, a decline in the straining effect, and competitive deposition between CDs and surfactant molecules. Additionally, SDBS increased the electrostatic repulsion of CDs and soil. Interestingly, in the divalent cation background electrolytes (i.e., Ca2+ or Cu2+), SDBS suppressed CDs’ mobility, whereas CTAB had the opposite effect. The transport-inhibited effect of SDBS was mainly due to anionic surfactant ion (DBS−) precipitation with metal cations and the formation of adsorbed SDBS-Cu2+/Ca2+-CDs complexes. The enhanced effect of CTAB resulted from the CTAB coating on soil grains, which suppressed the cation bridging between CDs and soil. Furthermore, the magnitude of the SDBS promotion effect was pH-dependent. Surprisingly, CTAB could inhibit CDs’ mobility at pH 9.0, owing to the binding cationic surfactant’s strong hydrophobicity effect on the soil surface. Moreover, the experimental breakthrough curves of CDs were well described using a two-site transport model. Overall, the observations obtained from this study shed light on the relative mobility of CDs with different surfactants in typical groundwater conditions.





Similar content being viewed by others
Data availability
The authors declare that all relevant data supporting the findings of this study are included in this article and its supplementary information files.
References
Alkan M, Karadas M, Dogan M, Demirbas O (2005) Adsorption of CTAB onto perlite samples from aqueous solutions. J Colloid Interface Sci 291:309–318
Astefanei A, Nunez O, Galceran MT, Kok WT, Schoenmakers PJ (2015) Aggregation behavior of fullerenes in aqueous solutions: a capillary electrophoresis and asymmetric flow field-flow fractionation study. Anal Bioanal Chem 407:8035–8045
Atay NZ, Yenigün O, Asutay M (2002) Sorption of anionic surfactants SDS, AOT and cationic surfactant hyamine 1622 on natural soils. Water Air Soil Pollut 136:55–68
Blunk D, Bierganns P, Bongartz N, Tessendorf R, Stubenrauch C (2006) New speciality surfactants with natural structural motifs. New J Chem 30:1705
Boakye-Ansah S, Khan MA, Haase MF (2020) Controlling surfactant adsorption on highly charged nanoparticles to stabilize bijels. J Phys Chem C 124:12417–12423
Bouchard D, Zhang W, Powell T, Rattanaudompol US (2012) Aggregation kinetics and transport of single-walled carbon nanotubes at low surfactant concentrations. Environ Sci Technol 46:4458–4465
Bouchard D, Zhang W, Chang X (2013) A rapid screening technique for estimating nanoparticle transport in porous media. Water Res 47:4086–4094
Bradford SA, Simunek J, Bettahar M, Van Genuchten MT, Yates SM (2003) Modeling colloid attachment, straining, and exclusion in saturated porous media. Environ Sci Technol 37:2242–2250
Bradford SA, Torkzaban S, Walker SL (2007) Coupling of physical and chemical mechanisms of colloid straining in saturated porous media. Water Res 41:3012–3024
Chrysikopoulos CV, Syngouna VI (2014) Effect of gravity on colloid transport through water-saturated columns packed with glass beads: modeling and experiments. Environ Sci Technol 48:6805–6813
Dai C, Shen H, Duan Y, You X, Lai X, Liu S, Zhang Y, Hon LK, Baek K, Tu Y, Zhou L, Xu D (2022) Transport of TiO2 and CeO2 nanoparticles in saturated porous media in the presence of surfactants with environmentally relevant concentrations. Environ Sci Pollut Res 29:9306–9317
Das A, Gude V, Roy D, Chatterjee T, De CK, Mandal PK (2017) On the molecular origin of photoluminescence of non-blinking carbon dot. J Phys Chem C 121:9634–9641
Das S, Mondal S, Ghosh S (2013) Physicochemical studies on the micellization of cationic, anionic, and nonionic surfactants in water–polar organic solvent mixtures. J Chem Eng Data 58:2586–2595
Ding H, Du F, Liu P, Chen Z, Shen J (2015) DNA-carbon dots function as fluorescent vehicles for drug delivery. ACS Appl Mater Inter 7:6889–6897
Duan WH, Wang Q, Collins F (2011) Dispersion of carbon nanotubes with SDS surfactants: a study from a binding energy perspective. Chem Sci 2:1407
Fan W, Jiang X, Lu Y, Huo M, Lin S, Geng Z (2015) Effects of surfactants on graphene oxide nanoparticles transport in saturated porous media. J Environ Sci 35:12–19
Fang J, Zhang K, Sun P, Lin D, Shen B, Luo Y (2016) Co-transport of Pb2+ and TiO2 nanoparticles in repacked homogeneous soil columns under saturation condition: effect of ionic strength and fulvic acid. Sci Total Environ 571:471–478
Frank BP, Sigmon LR, Deline AR, Lankone RS, Gallagher MJ, Zhi B, Haynes CL, Fairbrother DH (2020) Photochemical transformations of carbon dots in aqueous environments. Environ Sci Technol 54:4160–4170
Geng Y, Zhang L, Li Y, Cao Y, Tian S, Zhao Q, Chai X (2021) Effect of pulmonary surfactant on the dispersion of carbon nanoparticles. Colloids Surf A 629:127399
Glover AJ, Adamson DH, Schniepp HC (2012) Charge-driven selective adsorption of sodium dodecyl sulfate on graphene oxide visualized by atomic force microscopy. J Phys Chem C 116:20080–20085
Han JH, Jung SK (2020) Toxicity evaluation of household detergents and surfactants using zebrafish. Biotechnol Bioproc Eng 26:156–164
Han Z, Zhang F, Lin D, Xing B (2008) Clay minerals affect the stability of surfactant-facilitated carbon nanotube suspensions. Environ Sci Technol 42:6869–6875
Hari AC, Paruchuri RA, Sabatini DA, Kibbey TCG (2005) Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material. Environ Sci Technol 39:2592–2598
Hsieh AG, Korkut S, Punckt C, Aksay IA (2013a) Dispersion stability of functionalized graphene in aqueous sodium dodecyl sulfate solutions. Langmuir 29:14831–14838
Hsieh AG, Punckt C, Korkut S, Aksay IA (2013b) Adsorption of sodium dodecyl sulfate on functionalized graphene measured by conductometric titration. J Phys Chem B 117:7950–7958
Hussien SE, Elraies KA, Almansour A, Husin H, Ern L (2019) Experimental study on the use of surfactant as a fracking fluid additive for improving shale gas productivity. J Petrol Sci Eng 183:106426
Jiang Y, Yin X, Xi X, Guan D, Sun H, Wang N (2021) Effect of surfactants on the transport of polyethylene and polypropylene microplastics in porous media. Water Res 196:117016
Jiang Y, Zhang X, Yin X, Sun H, Wang H (2018) Graphene oxide-facilitated transport of Pb2+ and Cd2+ in saturated porous media. Sci Total Environ 631–632:369–376
Jin R, Lu T, Zhang H, Wang M, Wang M, Qi W, Qi Z, Li D (2021) Role of solution chemistry in the attachment of graphene oxide nanoparticles onto iron oxide minerals with different characteristics. Environ Sci Pollut Res 28:5126–5136
Kamrani S, Amiri V, Kamrani M, Baalousha M (2020) Transport of N-CD and pre-sorbed Pb in saturated porous media. Molecules 25:1–13
Kamrani S, Rezaei M, Kord M, Baalousha M (2018a) Co-transport and remobilization of Cu and Pb in quartz column by carbon dots. Sci Total Environ 626:995–1004
Kamrani S, Rezaei M, Kord M, Baalousha M (2018b) Transport and retention of carbon dots (CDs) in saturated and unsaturated porous media: role of ionic strength, pH, and collector grain size. Water Res 133:338–347
Katzourakis VE, Chrysikopoulos CV (2014) Mathematical modeling of colloid and virus cotransport in porous media: application to experimental data. Adv Water Resour 68:62–73
Katzourakis VE, Chrysikopoulos CV (2015) Modeling dense-colloid and virus cotransport in three-dimensional porous media. J Contam Hydrol 181:102–113
Katzourakis VE, Chrysikopoulos CV (2021) Modeling the transport of aggregating nanoparticles in porous media. Water Resour Res 57:e2020WR027946
Kaur H, Sareen S, Verma M, Vashisht A, Sharma A, Kataria R, Pawar SV, Mehta SK, Park J (2021) Effect of synthesis methods and conditions on properties and applications of carbon dots for the detection of potential water contaminants: a review. https://doi.org/10.1080/10408347.2021.1977608
Kelarakis A (2014) From highly graphitic to amorphous carbon dots: a critical review. MRS Energy Sustain 1:1–15
Khan MN, Zareen U (2006) Sand sorption process for the removal of sodium dodecyl sulfate (anionic surfactant) from water. J Hazard Mater 133:269–275
Khan S, Sharma A, Ghoshal S, Jain S, Hazra M, Nandi CK (2017) Small molecular organic nanocrystals resemble the properties of carbon nanodots. Chem Sci 9:175–180
Klimonda A, Kowalska I (2021) Membrane technology for the treatment of industrial wastewater containing cationic surfactants. Water Resour Ind 26:100157
Kmetz AA, Becker MD, Lyon BA, Foster E, Xue Z, Johnston KP, Abriola LM, Pennell KD (2016) Improved mobility of magnetite nanoparticles at high salinity with polymers and surfactants. Energ Fuel 30:1915–1926
Lanphere JD, Luth CJ, Walker SL (2013) Effects of solution chemistry on the transport of graphene oxide in saturated porous media. Environ Sci Technol 47:4255–4261
Levine LH, Garland JL, Johnson JV (2005) Simultaneous quantification of poly-dispersed anionic, amphoteric and nonionic surfactants in simulated wastewater samples using C18 high-performance liquid chromatography-quadrupole ion-trap mass spectrometry. J Chromatogr A 1062:217–225
Li J, Chen J, Lu T, Wang Y, Zhang H, Shang Z, Li D, Zhou Y, Qi Z (2019) Effects of low-molecular weight organic acids on the transport of graphene oxide nanoparticles in saturated sand columns. Sci Total Environ 666:94–102
Li Q, Ohulchanskyy TY, Liu R, Koynov K, Wu D, Best A, Kumar R, Bonoiu A, Prasad PN (2010) Photoluminescent carbon dots as biocompatible nanoprobes for targeting cancer cells in vitro. J Phys Chem C 114:12062–12068
Lin D, Tian X, Wu F, Xing B (2010) Fate and transport of engineered nanomaterials in the environment. J Environ Qual 39:1896–1908
Liang Y, Bradford SA, Simunek J, Heggen M, Vereecken H, Klumpp E (2013) Retention and remobilization of stabilized silver nanoparticles in an undisturbed loamy sand soil. Environ Sci Technol 47:12229–12237
Liu JC, Lien CY (2006) Dissolved air flotation of polishing wastewater from semiconductor manufacturer. Water Sci Technol 53:133–140
Liu L, Gao B, Wu L, Sun Y, Zhou Z (2015) Effects of surfactant type and concentration on graphene retention and transport in saturated porous media. Chem Eng J 262:1187–1191
Liu ML, Chen BB, Li CM, Huang CZ (2019) Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem 21:449–471
Liu W, Ren D, Wu J, Wang Z, Zhang S, Zhang X, Gong X (2021) Adsorption behavior of 2,4-DCP by rice straw biochar modified with CTAB. Environ Technol 42:3797–3806
Lu Y, Xu X, Yang K, Lin D (2013) The effects of surfactants and solution chemistry on the transport of multiwalled carbon nanotubes in quartz sand-packed columns. Environ Pollut 182:269–277
Meierhofer F, Dissinger F, Weigert F, Jungclaus J, Voss T (2020) Citric acid based carbon dots with amine type stabilizers: pH-specific luminescence and quantum yield characteristics. J Phys Chem C 124:8894–8904
Mohammed I, Afagwu CC, Adjei S, Kadafur IB, Jamal MS, Awotunde AA (2020) A review on polymer, gas, surfactant and nanoparticle adsorption modeling in porous media. Oil Gas Sci Technol 75:77
Mutreja V, Kumar A, Sareen S, Pathania K, Sandhu H, Kataria R, Pawar SV, Mehta SK, Park J (2022) Aggregation-induced quenching of carbon dots for detection of nitric oxide. Chem Select 7:e202200448
Oleszczuk P, Xing B (2011) Influence of anionic, cationic and nonionic surfactants on adsorption and desorption of oxytetracycline by ultrasonically treated and non-treated multiwalled carbon nanotubes. Chemosphere 85:1312–1317
Peng X, Yuan Y, Wang H, Liang C (2016) Aqueous stability and mobility of C60 complexed by sodium dodecyl benzene sulfonate surfactant. J Environ Sci 42:89–96
Petosa AR, Jaisi DP, Quevedo IR, Elimelech M, Tufenkji N (2010) Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions. Environ Sci Technol 44:6532–6549
Piispanen PS, Persson M, Claesson P, Norin T (2004) Surface properties of surfactants derived from natural products. Part 1: Syntheses and structure/property relationships—Solubility and emulsification. J Surfactants Deterg 7:147–159
Ramprasad C, Philip L (2016) Sorption of surfactants and personal care products in Indian soils. Int J Environ Sci Technol 14:853–866
Rao P, He M (2006) Adsorption of anionic and nonionic surfactant mixtures from synthetic detergents on soils. Chemosphere 63:1214–1221
Reckmeier CJ, Schneider J, Xiong Y, Hausler J, Kasák P, Schnick W, Rogach AL (2017) Aggregated molecular fluorophores in the ammonothermal synthesis of carbon dots. Chem Mater 29:10352–10361
Santhiya D, Dias RS, Shome A, Das PK, Miguel MG, Lindman B, Maiti S (2009) Role of linker groups between hydrophilic and hydrophobic moieties of cationic surfactants on oligonucleotide-surfactant interactions. Langmuir 25:13770–13775
Schneider J, Reckmeier CJ, Xiong Y, Seckendorff MV, Susha AS, Kasák P, Rogach AL (2016) Molecular fluorescence in citric acid based carbon dots. J Phys Chem C 121:2014–2022
Sciortino A, Cannizzo A, Messina F (2018) Carbon nanodots: a review-from the current understanding of the fundamental photophysics to the full control of the optical response. C-J Carbon Res 4:67
Seetha N, Hassanizadeh SM (2022) A two-way coupled model for the co-transport of two different colloids in porous media. J Contam Hydrol 244:103922
Song Y, Zhu S, Zhang S, Fu Y, Wang L, Zhao X, Yang B (2015) Investigation from chemical structure to photoluminescent mechanism: a type of carbon dots from the pyrolysis of citric acid and an amine. J Mater Chem C 3:5976–5984
Sparks DL (1995) Environmental Soil Chemistry. Academic Press, San Diego
Sun P, Zhang K, Fang J, Lin D, Wang M, Han J (2015) Transport of TiO2 nanoparticles in soil in the presence of surfactants. Sci Total Environ 527–528:420–428
Syngouna VI, Chrysikopoulos CV (2016) Cotransport of clay colloids and viruses through water-saturated vertically oriented columns packed with glass beads: gravity effects. Sci Total Environ 545–546:210–218
Tao S, Feng T, Zheng C, Zhu S, Yang B (2019) Carbonized polymer dots: a brand new perspective to recognize luminescent carbon-based nanomaterials. J Phys Chem Lett 10:5182–5188
TengkuMohd TA, Jaafar MZ (2018) Influences of alkaline and salinity on adsorption capacity of anionic sodium dodecyl sulfate surfactant. Asia Proc Soc Sci 2:19–23
Tong X, Shi S, Tong C, Iftikhar A, Long R, Zhu Y (2020) Quantum/carbon dots-based fluorescent assays for enzyme activity. TrAC-Trend Anal Chem 131:116008
Toride N, Leij FJ, van Genuchten MT (1999) The CXTFIT code for estimating transport parameters from laboratory or field tracer experiments. Version 2.0, Research Report No. 137, US, Salinity Laboratory, USDA, ARS, Riverside
Torkzaban S, Bradford SA, Wan J, Tokunaga T, Masoudih A (2013) Release of quantum dot nanoparticles in porous media: role of cation exchange and aging time. Environ Sci Technol 47:11528–11536
Tufenkji N, Elimelech M (2004) Deviation from the classical colloid filtration theory in the presence of repulsive DLVO interactions. Langmuir 20:10818–10828
Tufenkji N, Elimelech M (2005) Breakdown of colloid filtration theory: role of the secondary energy minimum and surface charge heterogeneities. Langmuir 21:841–852
Tummala NR, Morrow BH, Resasco DE, Striolo A (2010) Stabilization of aqueous carbon nanotube dispersions using surfactants: insights from molecular dynamics simulations. ACS Nano 4:7193–7204
Wang C, Wang R, Huo Z, Xie E, Dahlke HE (2020) Colloid transport through soil and other porous media under transient flow conditions—a review. Wires Water 7:e1439
Wang D, Paradelo M, Bradford SA, Peijnenburg WJ, Chu L, Zhou D (2011) Facilitated transport of Cu with hydroxyapatite nanoparticles in saturated sand: effects of solution ionic strength and composition. Water Res 45:5905–5915
Wang D, Su C, Liu C, Zhou D (2014) Transport of fluorescently labeled hydroxyapatite nanoparticles in saturated granular media at environmentally relevant concentrations of surfactants. Colloids Surf A 457:58–66
Wang F, Jia Z, Su W, Shang Y, Wang ZL (2019a) Adsorption of phenanthrene and 1-naphthol to graphene oxide and L-ascorbic-acid-reduced graphene oxide: effects of pH and surfactants. Environ Sci Pollut Res 26:11062–11073
Wang L, Huang Y, Kan AT, Tomson MB, Chen W (2012) Enhanced transport of 2,2′,5,5′’-polychlorinated biphenyl by natural organic matter (NOM) and surfactant-modified fullerene nanoparticles (nC60). Environ Sci Technol 46:5422–5429
Wang M, Song Y, Zhang H, Lu T, Chen W, Li W, Qi W, Qi Z (2021a) Insights into the mutual promotion effect of graphene oxide nanoparticles and tetracycline on their transport in saturated porous media. Environ Pollut 268:115730
Wang M, Yu C, Tang D, Chen J, Gao B (2019b) Effects of surfactant and electrolyte concentrations, cation valence, and temperature on graphene oxide retention and transport in saturated porous media. Water Air Soil Pollut 230:21
Wang M, Zhang H, Chen W, Lu T, Yang H, Wang X, Lu M, Qi Z, Li D (2021b) Graphene oxide nanoparticles and hematite colloids behave oppositely in their co-transport in saturated porous media. Chemosphere 265:129081
Xia T, Lin Y, Guo X, Li S, Cui J, Ping H, Zhang J, Zhong R, Du L, Han C, Zhu L (2019) Co-transport of graphene oxide and titanium dioxide nanoparticles in saturated quartz sand: Influences of solution pH and metal ions. Environ Pollut 251:723–730
Yang K, Zhu L, Xing B (2006) Enhanced soil washing of phenanthrene by mixed solutions of TX100 and SDBS. Environ Sci Technol 40:4274–4280
Zhang B, Cho M, Hughes JB, Kim JH (2009a) Translocation of C60 from aqueous stable colloidal aggregates into surfactant micelles. Environ Sci Technol 43:9124–9129
Zhang H, Lu T, Zhang R, Wang M, Krishnan S, Liu S, Zhou Y, Li D, Qi Z (2020) Effects of clay colloids on ciprofloxacin transport in saturated quartz sand porous media under different solution chemistry conditions. Ecotoxicol Environ Saf 199:110754
Zhang J, Zeng J, He M (2009b) Effects of temperature and surfactants on naphthalene and phenanthrene sorption by soil. J Environ Sci 21:667–674
Zhang L, Hou L, Wang L, Kan AT, Chen W, Tomson MB (2012) Transport of fullerene nanoparticles (nC60) in saturated sand and sandy soil: controlling factors and modeling. Environ Sci Technol 46:7230–7238
Zhang M, Bradford SA, Simunek J, Vereecken H, Klumpp E (2019) Co-transport of multi-walled carbon nanotubes and sodium dodecylbenzenesulfonate in chemically heterogeneous porous media. Environ Pollut 247:907–916
Zhang M, Zhu L (2010) Effect of SDBS–Tween 80 mixed surfactants on the distribution of polycyclic aromatic hydrocarbons in soil–water system. J Soil Sediment 10:1123–1130
Zhang P, Liu Y, Li Z, Kan AT, Tomson MB (2018) Sorption and desorption characteristics of anionic surfactants to soil sediments. Chemosphere 211:1183–1192
Zhou D, Wang D, Cang L, Hao X, Chu L (2011) Transport and re-entrainment of soil colloids in saturated packed column: effects of pH and ionic strength. J Soil Sediment 11:491–503
Zhou Y, Liu L, Shen Y, Wu L, Yu L, Liang F, Xi J (2017) Carbon dots promoted vanadium flow batteries for all-climate energy storage. Chem Commun 53:7565–7568
Zhu L, Li Z, Tian R, Li H (2019) Specific ion effects of divalent cations on the aggregation of positively charged goethite nanoparticles in aqueous suspension. Colloids Surf A 565:78–85
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Edit 52:3953–3957
Acknowledgements
The authors would like to express their gratitude to the NSFC-Shandong United Fund, the National Key Research and Development Program, and the Fundamental Research Funds for the Central Universities.
Funding
This project was supported by the NSFC-Shandong United Fund (U1906222), the National Key Research and Development Program (2019YFC1804104), the Fundamental Research Funds for the Central Universities (226–2022-00084), the Opening Foundation of Key Laboratory of Environment Remediation and Ecological Health (Zhejiang University), Ministry of Education (EREH202212), the Project Management of Innovation and Entrepreneurship Training Program for Henan Kaifeng College of Science Technology and Communication Students (KCCXSYLX-2022–080).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Zhichong Qi designed all experiments. Taotao Lu and Jiuyan Chen performed the experiments. Qiang Zhang performed the integrated data analysis. Mengli Zhang and YanXiang Li performed the chemical analysis. Zhichong Qi interpreted the data and wrote the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Kitae Baek
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, T., Chen, J., Zhang, Q. et al. Surfactant-mediated mobility of carbon dots in saturated soil: comparison between anionic and cationic surfactants. Environ Sci Pollut Res 30, 37622–37633 (2023). https://doi.org/10.1007/s11356-022-24878-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24878-6