Abstract
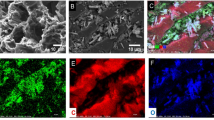
In this study, a novel in situ iron-loaded activated carbon (AFPAC) was prepared by a FeSO4/K2FeO4 impregnation and oxidation combination two-step supported on activated carbon for enhanced removal of Cr(VI) from aqueous solutions. Cr(VI) removal efficiency greatly increased by AFPAC more than 70% than that of fresh activated carbon (AC), which is due to rich iron oxides formed in situ and the synergistic effect between iron oxides and activated carbon. Cr(VI) adsorption behaviors on AFPAC under different water quality parameters were investigated. The maximum monolayer adsorption capacities for Cr(VI) by AFPAC are as high as 26.24 mg/g, 28.65 mg/g, and 32.05 mg/g at 25 °C, 35 °C and 45 °C at pH 4, respectively. Density functional theory (DFT) results showed that the adsorption energy of K2Cr2O7 on the surface of FeOOH was − 2.52 eV, which was greater than that on the surface of bare AC, and more charge transfer occurred during the adsorption of K2Cr2O7 on the surface of FeOOH, greatly promoting the formation of Cr = O-Fe. Cr(VI) removal by AFPAC included electrostatic attraction, redox reaction, coordinate complexation, and co-precipitation. Cr(VI) adsorption process on AFPAC consisted of the three reaction steps: (1) AFPAC was fast protonation and Cr2O72− would electrostatically attract to the positively charged AFPAC surface. (2) Cr2O72− was reduced into Cr2O3 by the carbons bond to the oxygen functionalities on activated carbon and the redox reaction process of FeSO4 and K2FeO4. (3) The inner-sphere complexes were formed, and adsorbed on AFPAC by iron oxides and then co-precipitation.











Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aljerf L (2018) High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: kinetics and equilibrium study. J Environ Manage 225:120–132
Almeida ACMO (2015) Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: kinetic and equilibrium studies. Chem Eng J 260:291–299
Bimbo N, Smith JP, Aggarwal H, Physick AJ, Pugsley A, Barbour LJ, Ting VP, Mays TJ (2021) Kinetics and enthalpies of methane adsorption in microporous materials AX-21, MIL-101 (Cr) and TE7. Chem Eng Res Des 169:153–164
Chen Y, Qian Y, Ma J, Mao M, Qian L, An D (2022) New insights into the cooperative adsorption behavior of Cr(VI) and humic acid in water by powdered activated carbon. Sci Total Environ 817:153081
Dan X, Luo Z, Dai M, Zhang M, Yue X, Xie S (2021) Oxidative degradation of p-chlorophenol by ferrate(VI): kinetics, intermediates and pathways. J Environ Chem Eng 9:105810
Deliyanni EA, Peleka EN, Matis KA (2009) Modeling the sorption of metal ions from aqueous solution by iron-based adsorbents. J Hazard Mater 172:550–558
Ding K, Zhou X, Hadiatullah H, Lu Y, Zhao G, Jia S, Zhang R, Yao Y (2021) Removal performance and mechanisms of toxic hexavalent chromium (Cr(VI)) with ZnCl2 enhanced acidic vinegar residue biochar. J Hazard Mater 420:126551
Huang J, Li Y, Jia X, Song H (2019) Preparation and tribological properties of core-shell Fe3O4@C microspheres. Tribol Int 129:427–435
Kahu SS, Shekhawat A, Saravanan D, Jugade RM (2016) Two fold modified chitosan for enhanced adsorption of hexavalent chromium from simulated wastewater and industrial effluents. Carbohydr Polym 146:264–273
Kang Y, Sun H, Gao B, Dang J, Zhang M, Li M, Dong J, Wu H, Zhang J, Guo Z (2022) Enhanced reduction of Cr(VI) in iron-carbon micro-electrolysis constructed wetlands: mechanisms of iron cycle and microbial interactions. Chemical Engineering Journal (Lausanne, Switzerland : 1996) 439
Kaur J, Kaur M, Ubhi MK, Kaur N, Greneche J (2021) Composition optimization of activated carbon-iron oxide nanocomposite for effective removal of Cr(VI)ions. Mater Chem Phys 258:124002
Kazak O (2021) Fabrication of in situ magnetic activated carbon by co-pyrolysis of sucrose with waste red mud for removal of Cr(VI) from waters. Environ Technol Innov 24:101856
Li B, Zhang L, Yin W, Lv S, Li P, Zheng X, Wu J (2021) Effective immobilization of hexavalent chromium from drinking water by nano-FeOOH coating activated carbon: Adsorption and reduction. J Environ Manage 277:111386
Li C, Li XZ, Graham N (2005) A study of the preparation and reactivity of potassium ferrate. Chemosphere 61:537–543
Li D, Ji G, Hu J, Hu S, Yuan X (2018) Remediation strategy and electrochemistry flushing & reduction technology for real Cr(VI)-contaminated soils. Chem Eng J 334:1281–1288
Li H, Zheng F, Wang J, Zhou J, Huang X, Chen L, Hu P, Gao J, Zhen Q, Bashir S, Liu JL (2020a) Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem Eng J 390:124513
Li J, Zhao J, Li B, Bu H, Yin W, Lv S, Wu J (2022a) Effective inactivation of Escherichia coli in aqueous solution by activated carbon-supported α-FeOOH as heterogeneous Fenton catalyst with high stability and reusability. J Environ Chem Eng 10(2):107347
Li X, Chen D, Li N, Xu Q, Li H, He J, Lu J (2020b) Efficient reduction of Cr(VI) by a BMO/Bi2S3 heterojunction via synergistic adsorption and photocatalysis under visible light. J Hazard Mater 400:123243
Li Z, Li T, Xing X, Bi Z, Qi P, Hu C, Xu G, Chen C, Ma K, Chen J (2022b) Inhibiting the increase of antibiotic resistance genes during drinking water distribution by superior microbial interface using Fe modified granular activated carbon. J Clean Prod 335:130225
Liang H, Song B, Peng P, Jiao G, Yan X, She D (2019) Preparation of three-dimensional honeycomb carbon materials and their adsorption of Cr(VI). Chem Eng J 367:9–16
Ling L, Zhang W (2017) Visualizing arsenate reactions and encapsulation in a single zero-valent iron nanoparticle. Environ Sci Technol 51:2288–2294
Liu W, Zhang J, Zhang C, Ren L (2012) Preparation and evaluation of activated carbon-based iron-containing adsorbents for enhanced Cr(VI) removal: Mechanism study. Chem Eng J 189–190:295–302
Ma R, Yan X, Pu X, Fu X, Bai L, Du Y, Cheng M, Qian J (2021) An exploratory study on the aqueous Cr(VI) removal by the sulfate reducing sludge-based biochar. Sep Purif Technol 276:119314
Maziarz P, Matusik J, Radziszewska A (2019) Halloysite-zero-valent iron nanocomposites for removal of Pb(II)/Cd(II) and As(V)/Cr(VI): Competitive effects, regeneration possibilities and mechanisms. J Environ Chem Eng 7:103507
Michael Chika Egwunyenga TCE (2021) Activated multi-walled carbon nanotubes decorated with zero valent nickel nanoparticles for arsenic, cadmium and lead adsorption from wastewater in a batch and continuous flow modes. J Hazard Mater 423(5):126993
Moghadam HNMA (2016) Surfactant modified montmorillonite as a low cost adsorbent for4-chlorophenol: equilibrium, kinetic and thermodynamic study. J Taiwan Inst Chem E 59:244–251
Nadra R, Aljerf L (2019) Developed greener method based on MW implementation in manufacturing CNFs. Int J Nanomanuf 15:269
Nasseh N, Khosravi R, Rumman GA, Ghadirian M, Eslami H, Khoshnamvand M, Al-Musawi TJ, Khosravi A (2021) Adsorption of Cr(VI) ions onto powdered activated carbon synthesized from Peganum harmala seeds by ultrasonic waves activation. Environ Technol Innov 21:101277
Peng Z, Zhao H, Lyu H, Wang L, Huang H, Nan Q, Tang J (2018) UV modification of biochar for enhanced hexavalent chromium removal from aqueous solution. Environ Sci Pollut Res Int 25:10808–10819
Rashtbari Y, Sher F, Afshin S, Hamzezadeh A, Ahmadi S, Azhar O, Rastegar A, Ghosh S, Poureshgh Y (2022) Green synthesis of zero-valent iron nanoparticles and loading effect on activated carbon for furfural adsorption. Chemosphere 287:132114
Roy Choudhury P, Majumdar S, Sahoo GC, Saha S, Mondal P (2018) High pressure ultrafiltration CuO/hydroxyethyl cellulose composite ceramic membrane for separation of Cr (VI) and Pb (II) from contaminated water. Chem Eng J 336:570–578
Samira Norouzi MHVA (2018) Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresource Technol 258:48–56
Sun C, Chen T, Huang Q, Zhan M, Li X, Yan J (2020) Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: performance and mechanism. Chem Eng J 380:122519 (Lausanne, Switzerland : 1996)
Sun G, Fu F, Yu G, Yu P, Tang B (2021) Migration behavior of Cr(VI) during the transformation of ferrihydrite-Cr(VI) co-precipitates: the interaction between surfactants and co-precipitates. Sci Total Environ 767:145429
Tadjenant Y, Dokhan N, Barras A, Addad A, Jijie R, Szunerits S, Boukherroub R (2020) Graphene oxide chemically reduced and functionalized with KOH-PEI for efficient Cr(VI) adsorption and reduction in acidic medium. Chemosphere 258:127316
Taleb K, Markovski J, Milosavljević M, Marinović-Cincović M, Rusmirović J, Ristić M, Marinković A (2015) Efficient arsenic removal by cross-linked macroporous polymer impregnated with hydrous iron oxide: Material performance. Chem Eng J 279:66–78
Tu B, Chen H, Xue S, Deng J, Tao H (2021) Ultrafast and efficient removal of aqueous Cr(VI) using iron oxide nanoparticles supported on Bermuda grass-based activated carbon. J Mol Liq 334:116026
Wan S, Li Y, Cheng S, Wu G, Yang X, Wang Y, Gao L (2022) Cadmium removal by FeOOH nanoparticles accommodated in biochar: effect of the negatively charged functional groups in host. J Hazard Mater 421:126807
Wang L, Bolan NS, Tsang D, Hou D (2020) Green immobilization of toxic metals using alkaline enhanced rice husk biochar: effects of pyrolysis temperature and KOH concentration. Sci Total Environ 720:137584
Xu J, Gao N, Deng Y, Xia S (2013) Nanoscale iron hydroxide-doped granular activated carbon (Fe-GAC) as a sorbent for perchlorate in water. Chem Eng J 222:520–526
Xu J, Gao N, Zhao D, Chu W, He G, Chen P (2016) Enhanced iron efficiency of Fe-impregnated granular activated carbon (Fe-GAC) for arsenate removal via Fe(II)-H2O2 method. J Taiwan Inst Chem E 67:443–452
Yang G, Wang J, Zhang H, Jia H, Zhang Y, Gao F (2021) New insight into quinones triggered ferrate in-situ synthesized polynuclear Fe-hydroxyl complex for enhancing interfacial adsorption in highly efficient removal of natural organic matter. Sci Total Environ 770:144844
Yanhao Zhang YWHZ (2020) Recycling spent lithium-ion battery as adsorbents to remove aqueous heavy metals: adsorption kinetics, isotherms, and regeneration assessment. Resour Conserv Recycl 156:104688
Yu J, Jiang C, Guan Q, Ning P, Gu J, Chen Q, Zhang J, Miao R (2018) Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 195:632–640
Yu Y, Wu K, Xu W, Chen D, Fang J, Zhu X, Sun J, Liang Y, Hu X, Li R, Fang Z (2021) Adsorption-photocatalysis synergistic removal of contaminants under antibiotic and Cr(VI) coexistence environment using non-metal g-C3N4 based nanomaterial obtained by supramolecular self-assembly method. J Hazard Mater 404:124171
Zhao J, Liu Y, Wang Q, Fu Y, Lu X, Bai X (2018) The self-catalysis of ferrate (VI) by its reactive byproducts or reductive substances for the degradation of diclofenac: kinetics, mechanism and transformation products. Sep Purif Technol 192:412–418
Funding
This study was supported by Natural Science Foundation of Science and Technology Department of Anhui Province (No.2008085QE242 and 2208085Y18), the Open Project of State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (No.ES201917), Engineering Research Center of biomembrane water purification and utilization technology (BWPU2020KF08), and National Natural Science Foundation of China (51878001).
Author information
Authors and Affiliations
Contributions
Yanli Kong: conceptualization, methodology, investigation, review, and editing.
Zhiyan Huang: data curation, formal analysis, visualization, and writing.
Hangyu Chu: formal analysis, visualization, and writing.
Yaqian Ma: formal analysis and data curation.
Jiangya Ma: resources, writing—review and editing, supervision, and data curation.
Yong Nie: resources, writing—review and editing and supervision.
Lei Ding: resources, writing—review and editing.
Zhonglin Chen: writing—review and editing.
Jimin Shen: writing— review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
I agree to participate in this called Enhanced removal of aqueous Cr(VI) by the in situ iron-loaded activated carbon through a facile impregnation with Fe(II) and Fe(VI) two-step method: mechanism study. Signature of participants: Yanli Kong, Zhiyan Huang, Hangyu Chu, Yaqian Ma, Jiangya Ma (corresponding author), Yong Nie, Lei Ding, Zhonglin Chen, Jimin Shen.
Consent for publication
The author agrees to publication and the copyright to the article is transferred to the Journal of Environmental Science and Pollution Research if and when the article is accepted for publication. The author warrants that his/her contribution is original and that he/she has full power to make this grant. The author signs for and accepts responsibility for releasing this material on behalf of any and all co-authors. The copyright transfer covers the exclusive right to reproduce and distribute the article, including reprints, translations, photographic reproductions, microform, electronic form, or any other reproductions of similar nature. After submission of the agreement signed by the corresponding author, changes of authorship or in the order of the authors listed will not be accepted.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dear Editor,
We the undersigned declare that this manuscript entitled “ Enhanced removal of aqueous Cr(VI) by the in situ iron loaded activated carbon through a facile impregnation with Fe(II) and Fe(VI) two step method: Mechanism study” is original, has not been published before and is not currently being considered for publication elsewhere.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, Y., Huang, Z., Chu, H. et al. Enhanced removal of aqueous Cr(VI) by the in situ iron loaded activated carbon through a facile impregnation with Fe(II) and Fe(VI) two step method: Mechanism study. Environ Sci Pollut Res 30, 38480–38499 (2023). https://doi.org/10.1007/s11356-022-24876-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24876-8