Abstract
The pollution of the surface waters by pharmaceuticals and personal care products (PPCPs) has attracted worldwide attention, but data regarding their occurrence and potential risks for the aquatic biota on tropical coastal rivers of South America are still scarce. In this context, the occurrence and the preliminary ecological risk assessment of eleven pharmaceuticals of various therapeutic classes (including cocaine and its primary metabolite, benzoylecgonine) were investigated, for the first time, in five rivers of São Paulo, southeast Brazil, covering a coastline of about 140 km, namely Perequê River, Itinga River, Mongaguá River, Itanhaém River and Guaraú River. Although these five rivers are born in well-preserved areas of the Atlantic rainforest biome, on its way to sea and when they cross the urban perimeter, they receive untreated sewage discharges containing a complex mixture of contaminants. In addition, a “persistence, bioaccumulation and toxicity” (PBT) approach allowed to pre-select the priority PPCPs to be monitored in this coastline. Identification of several PPCPs in the samples was done using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Ten PPCPs were successfully quantified in all five rivers, namely caffeine (9.00–560.00 ng/L), acetaminophen (<LOQ–22.24 ng/L), benzoylecgonine (0.30–14.93 ng/L), atenolol (0.12–13.22 ng/L), losartan (0.10–8.42 ng/L), diclofenac (0.76–3.93 ng/L), cocaine (0.05–3.22 ng/L), furosemide (<LOQ–3.16), carbamazepine (0.04–0.50 ng/L) and orphenadrine (<LOQ–0.14 ng/L). From an ecological risk perspective, caffeine, acetaminophen and losartan can be considered as priority PPCPs because they showed low to moderate risks to algae, crustacean and fishes. However, using the PBT approach, carbamazepine and orphenadrine were also classified as priority compounds, followed by furosemide, acetaminophen, cocaine and losartan (all in second position) and caffeine, atenolol, diclofenac and benzoylecgonine (all in third position). This study provides valuable information to reinforce the importance of continuous monitoring of the coastal rivers of South America (containing PPCPs and illicit drugs) whose diffuse loads flow continuously into the marine ecosystems. Furthermore, ecotoxicological studies (especially with tropical marine organisms) to assess the long-term toxicity of these bioactive compounds are urgent.
Similar content being viewed by others
Introduction
All over the world, namely in the USA, Europe and Asia, several studies have been conducted to better understand the widespread occurrence, behaviour, fate and potential ecological risks of pharmaceuticals and personal care products (PPCPs) in the coastal areas (Biel-Maeso et al. 2018; Dafouz et al. 2018; Fernández-Rubio et al. 2019b). Although PPCPs are not routinely monitored and under environmental regulation in many countries (Čelić et al. 2019; Maasz et al. 2019), several works have shown that these environmental stressors may have deleterious effects on the aquatic biota at vestigial concentrations (i.e. nanogram to microgram per litre), such as behavioural changes, mortality, immobilisation, growth and reproduction inhibition, endocrine disruption, genotoxicity and carcinogenicity (Adams et al. 2020; Hamid et al. 2021; Reque et al. 2021).
However, compared with the rest of the world, reports on the occurrence, potential ecotoxicological risks and prioritization of widely used PPCPs and illicit drugs of highest environmental concern on the coastal aquatic ecosystems of South America are still scarce (Maranho et al. 2021; Mello et al. 2022; Pusceddu et al. 2022). For instance, the data about the occurrence and the ecological risk of PPCPs in the State of São Paulo, Brazil, an area which has about 880 km of coastline, covers 16 municipalities (about 2.0 million inhabitants) and has about 600 urban drainage channels whose waters flow into 290 tourist beaches, is very limited and represents a cause for environmental concern and a significant research gap (Roveri et al. 2020a, 2020b, 2021). Two recent works pinpointed that an important source of PPCPs and illicit drugs to the aquatic ecosystems of the São Paulo coastline is the urban channels of Guarujá and Santos cities, where a preliminary ecological risk assessment revealed that caffeine, acetaminophen, diclofenac, losartan and valsartan presented moderate to severe risks to sensitive aquatic organisms at maximum measured environmental concentrations (Roveri et al. 2020a, 2021).
In addition to these 600 artificial urban channels (registered by the Environmental Agency of São Paulo), the São Paulo coastline has several streams and coastal rivers, which are under strong anthropic pressure and carry out the diffuse loads of neighbouring cities directly into the South Atlantic Ocean, namely the Perequê River (located on Perequê Beach, Guarujá City), Itinga River (located on Solemar Beach, Praia Grande City), Mongaguá River (located on Central Beach, Mongaguá City), Itanhaém River (located on Centro Beach, Itanhaém City) and Guaraú River (located on Guaraú Beach, Peruíbe City) (SMA/CPLEA 2016; SMA/CPLA 2018; Cetesb 2020). These rivers are born in well-preserved areas of the Atlantic rainforest, a Brazilian Biome that harbours outstanding species richness and levels of endemism, representing one of the most biodiverse regions on Earth (São Paulo 2008; Moreira et al. 2017, 2019). However, on their way to the sea, these watercourses cross the urban perimeter being contaminated by different pollution diffuse sources that include untreated sewage discharge and illegal disposal of domestic waste (Biudes and Camargo 2006; Ferreira and Petrere 2009; Moreira et al. 2019). In this context, although the continental activities strongly influence the marine environment through river discharges (Dufresne et al. 2020), as far as it is known, no study has been dedicated to evaluate the occurrence and ecological risks of these emerging pollutants discharged into the coastal rivers that flow to the São Paulo coastline, an area of socio-economic importance, ecological relevance and intense human recreation. Moreover, given the vast number of PPCPs recently detected in the coast of São Paulo by several authors (Pereira et al. 2016; Cortez et al. 2018; Roveri et al. 2020a, 2021), efforts are needed to prioritize the chemicals of highest concern to ensure that resource-limited monitoring campaigns collect the most important data regarding the potential threats to the environmental and public health.
Against this backdrop, the objectives of this study are (i) to investigate, for the first time, the occurrence of PPCPs of various therapeutic classes (including cocaine and its primary metabolite, benzoylecgonine) in five coastal rivers of the São Paulo coastline; (ii) to use the maximum measured environmental concentrations (MECs) of these PPCPs to estimate the potential risks to aquatic non-target organisms, such as algae, crustacean and fishes; and (iii) to use the persistence, bioaccumulation and toxicity (PBT) criteria, to create a list of priority PPCPs, which may serve as a basis for focused monitoring of hazardous compounds in São Paulo coastline. The ultimate goal is that governmental agencies and policy-makers use this information to monitor and/or to manage the diffuse loads that flow into the aquatic ecosystems of the Brazilian coastal zone.
Materials and methods
Study site description and sample collection
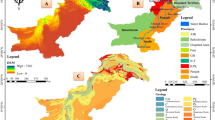
This study was carried out in the Metropolitan Region of Baixada Santista (MRBS), an area that occupies about 2800 km2 of São Paulo coastline, englobing nine municipalities: Bertioga, Cubatão, Guarujá, Itanhaém, Mongaguá, Peruíbe, Praia Grande, Santos and São Vicente (Cavalcanti et al. 2017; Moreira et al. 2017; Ibge, 2019). In this area, the Metropolitan Human Development Index (HDI) is 0.777 and the gross domestic product (GDP) is R$47.3 billion (Moreira et al. 2017). Although 67% of the MRBS is environmentally preserved, it presents a high degree of urbanization (over 97%), with more than 2.0 million inhabitants, which corresponds to almost 4% of the total population of the State of São Paulo (Cavalcanti et al. 2017; Moreira et al. 2017; Ibge, 2019). MRBS presents a small seasonal variation of climatic characteristics, i.e. a humid subtropical climate and mild winters. Two main annual seasons are observed in the region: a rainy period (November to March) and a dry period (April to October). The mean annual precipitation and temperature reach 2183 mm and 22.5 °C, respectively (SMA/CPLEA 2016; SMA/CPLA 2018). Favoured by these climate conditions and endowed by an ecosystem diversity, it is possible to find along this coastline, mangroves, rocky shorelines, sandbanks and estuaries, besides beaches which provide good conditions for tourism throughout the year (Moreira et al. 2017, 2019). Due to the tourism, mainly during the Brazilian summer, the MRBS coexists with an expressive floating population, which almost double in the high season (between December and March) (SMA/CPLEA 2016; SMA/CPLA 2018; Cetesb 2020). In this work, five coastal rivers of the MRBS were selected, along a coastal stretch of about 140 km, namely (i) Perequê River (that is draining into Perequê Beach, Guarujá City; 23° 56′ 05″ S; 46° 10′ 51″ W), (ii) Itinga River (that is draining into Solemar Beach, Praia Grande City; 24° 4′ 52″ S; 46° 35′ 55″ W), (iii) Mongaguá River (that is draining into Central Beach, Mongaguá City; 24° 05′ 13″ S; 46° 37′ 44″ W), (iv) Itanhaém River (that is draining into Centro Beach, Itanhaém City; 24° 11′ 08″ S; 46° 47′ 15″ W) and (v) Guaraú River (that is draining into Guaraú Beach, Peruíbe City; 24° 26′ 29″ S;47° 04′ 15″ W) (SMA/CPLEA 2016; SMA/CPLA 2018; Ibge 2019). For more details of the different characteristics regarding use and land occupation of these beaches, see Fig. 1 and Table S1.
A Map showing the location of São Paulo State (in blue colour) in Brazil. B Location of the Metropolitan Region of Baixada Santista, including the area of the study (black box). C Details of the five riverine collections points (in yellow colour), along the coastline of São Paulo, southeast Brazil, covering an extension of 140 km, namely D Perequê River (point 1), Itinga River (point 2), Mongaguá River (point 3), Itanhaém River (point 4) and Guaraú River (point 5)
Water sampling took place during the dry low-tourist season at the mouth of the rivers. The samples were collected during the low-tide periods, and no rainfall was recorded 48 h prior to collection. Moreover, weekends were avoided, to include only the disposal of PPCPs and illicit drugs by the resident population of these five cities. Thus, for all locations, sampling took place on Thursday, 10th of June 2021. Discrete water samples (1 L) were collected manually (at 30 cm depth in the water column) from each site with a stainless steel bucket which had been pre-cleaned with nitric acid, methanol and distilled water, and then rinsed twice with water from the sampling site before collection. After collection, water samples were stored in amber bottles, also previously cleaned with nitric acid and methanol, and rinsed with distilled water to eliminate any trace of possible contaminants. All samples were kept at 4 °C, and target PPCPs were extracted from water samples within 4 days of collection (USEPA 2007).
Preparation and analysis of pharmaceutical compounds
Chemical and standards
Selection of PPCPs was based on priority pollutant lists developed by the United States Environmental Protection Agency (USEPA), as well as the European Union (EU) (USEPA 2007, 2017; Zhou et al. 2019). Other selection criteria include reported annual consumption in Brazil (CMED 2019); frequent environmental occurrence in São Paulo coastal zone, namely in marine sewage discharges and urban drainage channels (Pereira et al. 2016; Cortez et al. 2018; Fontes et al. 2019; Fontes et al. 2020; Roveri et al. 2020a, 2020b); known persistence and toxicity to aquatic organisms (Pereira et al. 2016; Roveri et al. 2020a, 2021); and because analytical standards and valid laboratorial protocols already exist (Pereira et al. 2016; Roveri et al. 2020a, 2020b, 2021). Chemicals and analytical reagents such as nitric acid and sulphuric acid were purchased from Merck (Darmstadt, Germany). Grade solvents used in high-performance liquid chromatography (HPLC) and liquid chromatography with tandem mass spectrometry (LC-MS/MS), such as acetonitrile, methanol and isopropanol, were acquired from Sigma-Aldrich (MA, USA). Mobile phase additives, LC-MS grade formic acid and ammonium acetate, were acquired from Sigma-Aldrich and Merck, respectively. Analytical standards (with purity grade > 98%) of carbamazepine, caffeine, acetaminophen, diclofenac, orphenadrine, atenolol and losartan were acquired from Sigma-Aldrich. Chlortalidone and furosemide were acquired from Higroton® (Novartis, Switzerland), and cocaine and benzoylecgonine were acquired from Cerilliant (TX, USA).
Sample preparation
The extraction technique used was adopted from Roveri et al. (2020a, 2021). Prior to extraction, the following procedures were adopted: (i) the pH of each sample was adjusted to 7.0 ± 0.5 using a hydrochloric acid solution (1 M), (ii) samples were filtered through a cellulose filter paper (Whatman® GF/C glass microfiber filters, diameter 47 mm, particle retention 1.2 μm; Merck, Darmstadt, Germany), (iii) the filters were washed with 2 mL of methanol (Sigma-Aldrich, St. Louis, USA) and (iv) at the end, the methanol extract collected was then combined to the filtered sample. The solid-phase extraction was performed using SPE Chromabond HR-X cartridges (200 mg, 3 mL; Macherey-Nagel, Duren, Germany). The cartridges were pre-conditioned with methanol (5 mL) and ultrapure water (5 mL) (Milli-Q®; Merck, Darmstadt, Germany). Thereafter, they were loaded with 1 L of the filtered sample combined with the methanol from filter washings. The cartridges were then dried under vacuum for 30 min, and the elution was performed twice using 5 mL of methanol and 5 mL of acetone. After the extraction, the samples were dried under a nitrogen flow (at 50 °C) and eluted with water/acetonitrile (95:5 v/v) prior to mass spectrometry analysis. In the laboratory, each water sample was analysed in triplicate using LC-MS/MS. A concentration factor (1/1000) was used to obtain the final concentrations, and individual average results were expressed in ng/L (Table S2).
LC-MS/MS analysis
LC-MS/MS analytical procedures were validated by Shihomatzu (2015) and fully described by Roveri et al. (2021). Briefly, an aliquot of 10 μL of the water sample was analysed by HPLC Agilent 1260 (Agilent™, Germany) combined with a mass spectrometer hybrid triple quadrupole/LIT instrument (3200 QTRAP®, linear ion trap) (AB Sciex, Ontario, Canada). The samples were analysed using an Agilent Zorbax Eclipse XDB-C18 column (50 × 4.6 mm ID, 1.8 μm column at 25 °C). The eluent flow was 0.7 mL/min, and the mobile phase for positive mode analysis was 0.1% formic acid (Sigma-Aldrich; LC-MS grade) in water (solvent A) and acetonitrile (solvent B) (J.T. Baker, Philipsburg, NJ, USA). A linear gradient of 0.7 mL/min was used, starting with a mixture of solvent A (95%) and solvent B (5%). The percentage of solvent A was decreased linearly from 95 to 5% over 5 min, and this condition was maintained for 1 min. This mixture was then returned to initial conditions over 2 min, and the analytes were detected and quantified using the electrospray ionisation (ESI) and multiple reaction monitoring (MRM), with the selection of a precursor ion and two ion products to quantify and qualify each compound. The data were recorded and processed using the Analyst 1.5.2 software (AB Sciex). The MRM parameters for positive and negative ion modes, limit of detection (LOD) and limit of quantification (LOQ) are shown in Table S2.
Ecological risk assessment
The ecological risk assessment methodology followed the works of Roveri et al. (2020a, 2021). The risk quotient (RQ) for three different aquatic organisms (algae, crustaceans and fishes) was calculated following the equation RQ = MEC/PNEC, in which MEC is the maximum measured environmental concentration, and PNEC is the predicted no effect concentration, both expressed in ng/L. The PNEC values for the acute and chronic toxicity data were obtained from peer-reviewed publications by performing searches in the Ecotoxicology Database (ECOTOX) (USEPA 2019), as well as in other literature sources using the PubMed database (see Table S3). Specifically, PNEC values were obtained from short-term (lethal concentration 50 (LC50) or median effective concentration (EC50)) and long-term (no observed effect concentration (NOEC)) toxicological endpoints. In the absence of NOEC, the lowest observed effect concentration (LOEC) or, in alternative, the 10% effective concentration (EC10) was used, when available. However, when laboratory experimentally derived ecotoxicity data were not available, the short (L(E)C50) and long (chronic values (Chv), geometric mean of NOEC and LOEC, i.e. ChV=10^ ([log (NOEC × LOEC)]/2)) toxicological endpoints were estimated using the Ecological Structure Activity Relationships (ECOSAR, v 2.0) Program, developed by the USEPA (USEPA 2017a). An attempt was made to compile specifically PNEC data for marine coastal species. However, due to the strong land-sea interaction in this study area and the lack of marine toxicity data, the freshwater species were also taken into consideration in the present study (Roveri et al. 2020a, 2021). The PNEC values for the acute and chronic toxicity data were thereafter calculated by dividing each toxicological endpoint by an assessment factor (AF). According to the European Chemicals Bureau (ECB 2003) and the European Chemicals Agency (ECHA 2008) guidelines, for saltwater environments, an AF of 10,000 and 100 should be considered in short- and long-term data sets, respectively. The toxicological endpoints selected for the calculation of the PNECs are shown in Table S3. Finally, RQ was categorised into four levels: no (RQ < 0.01), low (0.01 ≤ RQ < 0.1), moderate (0.1 ≤ RQ < 1.0) and high (RQ ≥ 1.0) ecological risks to aquatic organisms (Hernando et al. 2006).
PBT criteria
The prioritization procedure applied to PPCPs detected in the aquatic ecosystems of the MRBS was adapted from Daouk et al. (2015). To each PPCP, a score has attributed, from 1 to 5, based on 3 criteria: persistence (P), bioaccumulation (B) and toxicity (T). For the persistence criteria, values of PPCP removal efficiency in sewage treatment plants (STPs) were estimated by the computational tool “STP total removal”, based on the original model developed by Clark et al. (1995), and it was used to verify the behaviour of a compound in an activated sludge sewage treatment plant (USEPA 2017b; Becker et al. 2021). The results are expressed in percentage of removal (%) (USEPA, 2017b). To verify the bioaccumulation potential of a compound, the “KOWWIN tool” was used to obtain the octanol-water partition coefficient (log Kow) (USEPA, 2017b). Both computational tools are predicted through Estimation Programs Interface Suite of models (EPI Suite program, version 4.11) developed by USEPA (USEPA, 2017b). Regarding the chronic toxicity, the PNEC data was obtained from ECOSAR, such as described in section “Ecological risk assessment” (USEPA, 2017a). The PBT final ranking was then obtained by the sum of the scores of the three criteria. For more details, see Table 3.
Results and discussion
Occurrence profile of the PPCPs in five coastal tropical rivers of southeast Brazil
To our knowledge, this is the first study to report PPCPs and illicit drugs in surface waters of coastal rivers of the MRBS, namely the Perequê River, Itinga River, Mongaguá River, Itanhaém River and Guaraú River. The occurrence of these compounds of different therapeutic classes, including illicit drugs, is shown in Table 1. With the exception of chlortalidone, not detected, the other 10 chemicals were successfully quantified (at least once), in one of the five rivers (Table 1). The MECs of these 10 compounds were mainly recorded in the Perequê River (Table 1). In the same way, the total concentrations of these 10 PPCPs (∑PPCPs) were also higher here (Table 1).
The Brazilian Atlantic rainforest is internationally recognised as one of the most biodiverse and threatened tropical forests in the world (São Paulo 2008; Moreira et al. 2017, 2019). The hereby selected rivers are born in the well-preserved environmental areas of this Biome that also include the protected areas of the cities of Peruíbe, Itanhaém, Mongaguá, Praia Grande and Guarujá (Fig. S1) (São Paulo 2008; Moreira et al. 2017, 2019). However, when these five rivers cross the urban perimeter, domestic sewage is released into these watercourses, constituting a main source of contaminants to these rivers (see more details in Table S1) (Biudes and Camargo 2006; Ferreira and Petrere 2009; Moreira et al. 2019). Indeed, in the case of Perequê Beach (where the higher concentrations of PPCPs were detected), it is estimated that about 10,000 of 25,000 inhabitants live in slums and/or in precarious housing (Roveri et al. 2020a, 2020b). Due to a lack of basic sanitation, a significant amount of the untreated sewage is discharged into the Perequê River, whose waters flow to the sea, compromising the public health and the environmental quality of the aquatic ecosystems (Roveri et al. 2020a, 2020b). On the contrary, the Guaraú River recorded the lowest concentration of PPCPs, probably because of the distance to the city downtown (low rates of urban occupation) and due to its proximity to the preserved areas of the state park of Juréia-Itatins (Fig. S1) (Roveri et al. 2012; Moreira et al. 2019). Consequently, as a result of numerous anthropic activities that take place along the Itanhaém, Mongaguá, Itinga and Perequê river hydrographic basins, these water resources have already been identified as a potential threat to the public health, as they are responsible for the introduction of allochthonous pathogenic microorganisms related to disease outbreaks (e.g. Escherichia coli and Enterococci), in areas of intense human recreation (see more details in Table S1) (Cetesb 2020). Additionally, the present study showed that these five coastal rivers are also responsible for the daily introduction of a complex mixture of chemical pollutants (containing PPCPs and illicit drugs) into the South Atlantic Ocean, in areas of extreme ecological relevance and intense human recreation. The MEC values recorded hereby are similar to the reported concentrations in other studies that took place in this region (e.g. around of discharge of the submarine outfalls and along of urban surface runoff of Santos and Guarujá cities) and, therefore, confirm the widespread presence of these PPCPs in the aquatic ecosystems of the MRBS (Fig. S2) (Pereira et al. 2016; Roveri et al. 2020a, 2021).
Moreover, the overall concentration of the stimulants (namely caffeine, cocaine and benzoylecgonine) in the coastal rivers of the MRBS was higher than the values reported in the surface waters in South America, Europa, Asia and Oceania (Table S2). However, this finding is not surprising. Caffeine is consumed daily by Brazilians in a wide range of products, such as coffee, tea, chocolate and cola. Moreover, caffeine is also used as an active ingredient of a variety of PPCPs (e.g. acetaminophen and orphenadrine) (Quadra et al. 2019). After consumed, about 10% of caffeine is excreted through human urine and faeces (Machado et al. 2016). Indeed, caffeine may enter aquatic ecosystems through irregular wastewater disposal, directly into household sinks, toilets and/or urban trash (Dafouz et al. 2018; Quadra et al. 2019; Korekar et al. 2019). Consequently, caffeine is being quantified worldwide in different aquatic environmental matrices (including the ecosystems of the MRBS), suggesting its use as a potential domestic sewage tracer, due to its widespread occurrence, environmental persistence and high concentration (Fig. S2) (Zhou et al. 2019; Roveri et al. 2020a, 2021). Regarding the abusive consumption of cocaine (a serious worldwide problem), recent data indicate that Brazil is the second world largest consumer of this psychoactive stimulant, and therefore, it is a serious social and public health problem (UNODC 2020). The MEC profile of cocaine (COC) and its primary metabolite, benzoylecgonine (BE) obtained hereby, is similar to those obtained by other studies performed in surface waters worldwide (i.e. concentrations of BE > COC: Table S4). This is because BE is the metabolite most excreted in human urine (around 45% of the consumed dose), and only about 9% of the administered dose of COC is excreted through urine in the original form (González-Mariño et al. 2019; Maasz et al. 2019; Maranho et al. 2021). Moreover, ecotoxicity and environmental persistence of this metabolite is equal or higher than that of the parent compound (Fernández-Rubio et al. 2019a; Maasz et al. 2019; Maranho et al. 2021). Therefore, as noted for caffeine, the relationship between COC and BE also is a reliable marker for domestic sewage contamination of the aquatic compartment (Roveri et al. 2020a, 2021).
Although the concentrations recorded for acetaminophen, diclofenac and orphenadrine in the five rivers of the MRBS were lower than those found in other international studies, the detection frequency of these compounds is consistent with the high non-prescribed sale of these drugs worldwide (Table 1 and S4) (Deen and Von Seidlein 2019; De Andrade Aragão et al. 2020; de Freitas and Radis-Baptista 2021). In Brazil, the pharmacies and drugstores commonly sell acetaminophen, diclofenac and orphenadrine without medical prescription, which means that the consumption of these drugs is not controlled (Abrafarma 2017; CMED 2019; de Freitas and Radis-Baptista 2021). In the case of the MRBS (region with approximately 2.0 million inhabitants), the high consumption of these analgesic and anti-inflammatory drugs can be enhanced, since there are around 6500 pharmacies and drugstores in this region (Abrafarma 2017; CMED 2019; Ibge 2019). Furthermore, self-medication of these PPCPs is a common practice among the Brazilian population (Arrais et al. 2016; Mello et al. 2022). For instance, acetaminophen was reported as the third most consumed drug in the Metropolitan Region of São Paulo, region bordering of the MRBS and with the highest gross domestic product in Brazil; this drugs is widely recommended for treatment against the fevers caused for dengue, Chikungunya and Zika (diseases still uncontrolled in Brazil), besides the fever caused by COVID-19 (disease that has already affected more than 20 million Brazilians) (Deen and Von Seidlein 2019; Scavone et al. 2020; de Freitas and Radis-Baptista 2021). Moreover, acetaminophen and diclofenac (an anti-inflammatory broad-spectrum drugs) can be used to reduce the pain and inflammation caused for osteoarthritis and rheumatoid arthritis (disease that affects about 2 million Brazilians) (Abrafarma 2017; CMED 2019). On the other hand, orphenadrine is recommended for the treatment of migraine, a disabling neurologic condition that affects a population of about 30 million Brazilians (Abrafarma 2017; CMED 2019). This high consumption of acetaminophen, diclofenac and orphenadrine raises environmental concerns. Indeed, during the oral administration of acetaminophen and diclofenac (through tablets), about 60% and 15% of the doses, respectively, are not absorbed by the human body, and therefore, they are released into the aquatic ecosystems through urine and/or faeces (Bouissou-Schurtz et al. 2014; USEPA 2017a).
The high consumption of antihypertensive and diuretic drugs, namely losartan and furosemide (including the β-blocker atenolol), is also a subject of great concern in Brazil, where about 70 million inhabitants are hypertensive (Ribeiro et al. 2016; Hanlon et al. 2017; McNally et al. 2019). For instance, the prescription of losartan to treat high blood pressure and heart failure became a usual practice in Brazil, being the sixth best-selling drug at the Metropolitan Region of São Paulo (region with approximately 21.5 million inhabitants) (CMED 2019; De Andrade Aragão et al. 2020). Furthermore, the age profile of the population of the MRBS may reflect the higher consumption rate of these PPCPs, because the consumption of multiple pharmaceuticals (polytherapy) is usually associated with an elderly population (Secoli et al. 2010; Lacorte et al. 2017; O’Flynn et al. 2021). In the MRBS, the people aged more than 60 years old represent approximately 16.2% of the population (slightly higher than the State of São Paulo: 15%) (Ibge 2019). Therefore, since the polytherapy for the treatment of hypertension (mainly for elderly people), it is a well-established practice in the country (e.g. losartan + furosemide and atenolol + furosemide) (Dos Santos et al. 2012; Ribeiro et al. 2016; Ibge, 2019), this may explain the high frequency of detection of antihypertensives, diuretic drugs and β-blocker in the five rivers of the MRBS (Table 1). Furthermore, pharmacies and drugstores have not adopted the unit-dose systems (UDSs) and policies of the reverse logistics (Pereira et al. 2017; De Andrade Aragão et al. 2020; de Freitas and Radis-Baptista 2021). This means that the population of Brazilians acquire more pills than prescribed because packages are sold sealed, which impedes fractioning (Abrafarma 2017; CMED, 2019; De Andrade Aragão et al. 2020). Moreover, although pharmacies and drugstores in the USA, Canada and Europe have adopted UDSs and roughly 50–90% of used PPCPs are collected via take-back schemes in these establishments, Brazil does not have yet taken-back schemes (Pereira et al. 2017; De Andrade Aragão et al. 2020; O’Flynn et al. 2021). Consequently, the lack of Brazilian public knowledge surrounding the appropriate disposal of unused or expired PPCPs can result in the intentional and direct release of these waste medicines in the sinks and/or toilets, which will end up in the aquatic ecosystems (Ribeiro et al. 2016; Hanlon et al. 2017; McNally et al. 2019).
However, except for atenolol, the MECs of losartan and furosemide quantified in the rivers of the MRBS were lower than the concentrations reported in worldwide surface waters (e.g. Europe, USA, Asia, Oceania and South America) (Table S4). Anyway, some physicochemical properties and/or degradation behaviours of these PPCPs deserve ecological attention (EMA 2006; EC 2015; USEPA 2017b). Furthermore, it is necessary to understand the ecological risks of these PPCPs to the non-target aquatic organisms, because the MECs of caffeine > acetaminophen > benzoylecgonine > diclofenac and atenolol were higher than 10.0 ng/L (Table 1), which is considered the threshold for the risk evaluation of pharmaceuticals in surface waters according to the European Medicines Agency (EMA 2006).
Ecological risk of the detected PPCPs
Using the PNEC from data available in the scientific peer-reviewed literature or estimated by the ECOSAR programme, the RQs in Perequê, Itinga, Mongaguá, Itanhaém and Guaraú rivers were calculated using the maximum MEC to evaluate the worst case of ecological risk for the aquatic biota (ECB 2003; EMA, 2006). For further details, see Table S3 that shows the complete data for the ten detected and quantified PPCPs in these five rivers. Figure 2 and Table 2 only show the summary data for the three compounds (namely caffeine, acetaminophen and losartan) that represent ecological potential risks in the MRBS.
Risk assessment data regarding the three compounds (i.e. caffeine, acetaminophen and losartan) that indicated the potential ecological threat for the aquatic biota in the five rivers of the Metropolitan Region of Baixada Santista, São Paulo, Brazil, namely Perequê River (point 1), Itinga River (point 2), Mongaguá River (point 3), Itanhaém River (point 4) and Guaraú River (point 5). Note that (i) all the risk quotients (RQs) were calculated, taking into consideration the maximum measured environmental concentrations (MECs) (in ng/L); (ii) the rivers were ordered (from top to bottom) according to the degree of risk, i.e. the plus symbol (+) indicates greater ecological risk, and the minus symbol indicates lower ecological risk; (iii) <LOQ limit of quantification. For further details, see Table 1, Table 2 and Table S3 that show the complete data for the ten detected and quantified pharmaceuticals and personal care products (PPCPs) in the study area
Caffeine is highly soluble in water (> 10,000 mg/L), shows low hydrophobicity (log Kow = 0.07) and has an estimated half-life in aquatic environment of about 1.5 day (USEPA 2017b). However, due to its widespread occurrence and high concentrations in surface waters (e.g. in the ecosystems of the MRBS; Fig. S1), it entered on the list of priority PPCPs in Europe (Zhou et al. 2019). In the MRBS, the MEC of the caffeine detected in the Perequê River (560 ng/L) deserves attention, because it indicated low to moderate risks for different trophic levels (Fig. 2 and Table 2). For instance, the MEC equal to that in the Perequê River suggested moderate risks for different trophic levels, namely in the rivers of Henarese Jaramae-Tajo and Guadalquivir (both in Spain) (Fernández et al. 2010; Robles-Molina et al. 2014) and in the urban channel of Santos (located on the MRBS) (Roveri et al. 2021). Moreover, previous studies shown that (i) chronic exposure to 500 ng/L of caffeine could alter the regenerative capacity of the annelid Diopatra neapolitana (Pires et al. 2016), and (ii) the molluscs Mytilus galloprovincialis have suffered lysosomal membrane destabilisation after exposure to caffeine concentrations of 500 ng/L (Capolupo et al. 2016).
Acetaminophen has been categorised as readily biodegradable (log Kow = 0.46), highly soluble (solubility 14,000 mg/L) and with a reported half-life of 20 days (Moermond et al. 2012). However, in Mongaguá, Itinga and Perequê rivers, the RQ of acetaminophen suggests low risk for crustacean and low to moderate risks for fish Danio rerio (Fig. 2 and Table 2). In this context, some ecotoxicological studies have also indicated potential risks of acetaminophen for fishes, namely an increase of mortality and developmental abnormalities in Danio rerio (Galus et al. 2013), but also oxidative stress problems in Oncorhynchus mykiss and Anguilla anguilla fishes (Ramos et al. 2014; Nunes et al. 2015). In the case of potential risks for crustaceans, previous studies in surface waters of Valencia, Spain (Vazquez-Roig et al. 2012), Lahore, Pakistan (Ashfaq et al. 2019), and Santos, Brazil (Roveri et al. 2021), indicated moderate risks of acetaminophen for Daphnia magna (RQ between 0.3 and 0.4).
The log Kow for losartan is greater than 4, and thus, this compound can persist in the aquatic environment, potentially bioaccumulate and/or can cause ecotoxicity (EMA 2006; EC 2015; USEPA 2017b). In the MRBS, the recorded levels of losartan raise environmental concern for Itanhaém, Mongaguá, Itinga and Perequê rivers, because the RQ indicate low to moderate risks for Daphnia magna (Fig. 2 and Table 2). Likewise, in the urban channels of Guarujá and Santos, the RQ obtained for losartan also suggested a moderate risk (for both acute and chronic exposures) for Daphnia (Roveri et al. 2020a, 2021). However, ecotoxicological studies with losartan, in non-target aquatic organisms, are still insufficient (Reque et al. 2021). Anyway, previous studies have reported toxic effects of losartan, but in “non-relevant” environmental concentrations (in the order of mg/L), in Desmodesmus subspicatus algae (Adams et al. 2020; Reque et al. 2021) and in three crustacean species, namely Daphnia similis, Ceriodaphnia dubia (Yamamoto et al. 2012) and Daphnia magna (Adams et al. 2020). Moreover, although information about the toxicity of losartan for marine tropical species is still poorly documented, two recent studies detected risks of losartan to important seafood species in the coastal regions of Brazil: (i) cytotoxic effects for mussel Perna perna exposed to environmental realistic concentrations of losartan (up to 3000 ng/L) were observed (Cortez et al. 2018), and (ii) a study showed that sediment spiked with an environmental concentration of losartan (LOEC = 3 ng/g) affected the lysosomal stability of Mytella guyanensis adult bivalves (Pusceddu et al. 2022).
List of priority PPCPs in the MRBS (PBT criteria)
Although the hereby ecological risks for the other PPCPs, namely carbamazepine, cocaine, benzoylecgonine, atenolol, orphenadrine, diclofenac and furosemide, were non-existent (RQ ≤ 0.01) (Table S3) for the reported concentrations, some degradation behaviours and/or physicochemical properties of these compounds deserve attention (EMA 2006; EC 2015; USEPA 2017b). For instance, based on kinetic reaction rate (k biol), mechanism of action (MoA), enzyme modulation, adverse effects and log Kow, carbamazepine, atenolol, diclofenac and furosemide were considered barely degradable and poorly adsorbable (refractory to sewage secondary treatment) and, thus, were classified as priority substances for the monitoring in European surface waters (Besse and Garric 2008; EC 2015; Mathon et al. 2016; Arola et al. 2017; Biel-Maeso et al. 2018). In this context, considering the widespread presence of these compounds in the aquatic ecosystems of the MRBS (Fig. S2) (Pereira et al. 2016; Roveri et al. 2020a, 2021), a prioritization procedure, through PBT criteria, was applied (Table 3) (Daouk et al. 2015). Therefore, after the application of the endpoints “STP total removal” (persistence), “log Kow” (bioaccumulation) and “PNEC” (chronic toxicity) (USEPA 2017a, 2017b; Becker et al. 2021), results showed that (i) seven among the ten compounds detected in the MRBS have removal expectancy in STPs lower than 3%, namely caffeine, atenolol, furosemide, acetaminophen, cocaine, benzoylecgonine and carbamazepine (Table 4) (USEPA 2017b). This condition can be even more aggravated in the MRBS, because the municipalities of Santos and Guarujá only have a preliminary level of treatment; i.e. these STPs only perform a mechanical treatment (railing and screening for the removal of solids), followed by a chlorination step. The destination of these preconditioned sewages is submarine outfalls that daily dispose sewage into the Atlantic Ocean (Fig. S2) (Pereira et al. 2016; Roveri et al. 2020a, 2021); (ii) cocaine, carbamazepine and furosemide have moderate hydrophobicity (log Kow ≥ 2.3), and orphenadrine and diclofenac have high hydrophobicity (log Kow > 3.0) (Table 4). Therefore, all these compounds can potentially bioaccumulate and/or can cause ecotoxicity (Table S2) (EMA 2006; EC 2015; USEPA 2017b). In this context, recently, carbamazepine, diclofenac and furosemide were detected in fish and bivalve species widely consumed by Brazilian population (namely white mullet, snook, mullet, carib pointed venus and blue mussel) (Mello et al. 2022). Finally, the PBT approach showed that carbamazepine and orphenadrine are classified as priority substances for the monitoring in MRBS surface waters, followed by furosemide, acetaminophen, cocaine and losartan (all in second position) and caffeine, atenolol, diclofenac and benzoylecgonine (all in third position) (Table 4).
Ultimately, it is also important to note that PPCPs do not usually occur as a single and isolated chemical compound in the environment, but as complex mixtures (Lawrence et al. 2012; Bouissou-Schurtz et al. 2014). Consequently, once in the aquatic ecosystems, PPCP interactions may result in unpredicted deleterious effects in non-target organisms (Lawrence et al. 2012; Bouissou-Schurtz et al. 2014). In this context, the adverse effects posed by PPCP mixtures towards non-target organisms are an area of increasing scientific interest (Galus et al. 2013; Di Nica et al. 2016; Hamid et al. 2021). For instance, a study conducted in sewage waters from Ontario, Canada, showed significantly decreased Danio rerio embryo production after a 6-week exposure to a pharmaceutical mixture of acetaminophen, carbamazepine and other PPCPs (Galus et al. 2013). Another study accomplished in aquatic environments from Milan, Italy, showed that a combined effect of the carbamazepine, caffeine and diclofenac could be more severe to the metabolism of marine bacteria Aliivibrio fischeri than each drug individually (Di Nica et al. 2016). A recent study showed that environmentally relevant concentrations of a PPCP mixture (including carbamazepine, diclofenac and other compounds) found in Chongqing, China, induced developmental effects and metabolic dysfunction in both male and female of Danio rerio (Hamid et al. 2021). Therefore, in a real scenario, we cannot exclude a possible synergistic interaction between the several PPCPs quantified in this study (EMA 2006; Bouissou-Schurtz et al. 2014; USEPA 2017a).
Conclusion
In the Metropolitan Region of Baixada Santista, São Paulo coastline, Brazil, for the first time, ten PPCPs were positively detected in coastal five rivers, covering a coastal stretch of about 140 km, namely Perequê River, Itinga River, Mongaguá River, Itanhaém River and Guaraú River. Although these five rivers are born in pristine environments, when across the urban perimeter, they suffer anthropogenic contamination on their urban path to the sea. Consequently, the screening showed the widespread presence of several PPCPs, namely caffeine, acetaminophen, benzoylecgonine, atenolol, losartan, diclofenac, cocaine, furosemide, carbamazepine and orphenadrine, which were found in all the five sites. The highest concentrations of these PPCPs were detected in Perequê Beach, where, historically, sewage discharge stowaways usually occur, which lead to diffuse loads flowing directly into the marine ecosystem in areas of intense recreation. Given the widespread detection of these PPCPs in the Perequê River, the preliminary ecological risk assessment signalled great environmental concern, because the results suggest low to moderate ecotoxicological risks of caffeine, acetaminophen and losartan to algae, crustacean and fishes. This study recommends that the collection and treatment of the domestic sewage has to be more efficient, being necessary to eliminate the existing illegal connections in this beach. Itinga, Mongaguá and Itanhaém rivers had intermediate environmental risk, probably because of better levels of environmental sanitation. Finally, the Guaraú River did not show any environmental risk, probably because it is located nearby the preserved areas of the State Park of Juréia-Itatins and also shows the low rates of urban occupation.
Although the studies on the occurrence and ecological risk assessment of PPCPs in the coastline of South America are limited compared to the rest of the world, this study provided valuable insights about the current situation of these coastal tropical rivers of southeast Brazil, whose waters flow daily to the South Atlantic Ocean. Therefore, the results obtained in the present study complement already published data relating to the presence of PPCPs in Brazilian coastline and reinforce the need for (i) cooperation between stakeholders (namely manufacturers, pharmacists and consumers) to adopt the UDSs and policies of the reverse logistics for used PPCPs; (ii) inclusion of PPCPs and illicit drugs in the Brazilian environmental legislation as priority pollutants; (iii) further ecotoxicological studies (especially with tropical marine organisms) to assess the long-term toxicity of these bioactive compounds; (iv) resize the sewage treatment along the Brazilian coastline, including a level of treatment capable of removing, at least partially, PPCPs and illicit drugs; and (v) ultimately, a continuous environmental monitoring program is recommended, taking into account the precautionary and polluter pays principles, prioritizing those PPCPs more persistent, bioaccumulative and toxic, and has the greatest likelihood of harming the aquatic ecosystems of the Brazilian coastal zones.
References
Abrafarma. Brazilian Association of Pharmacy and Drugstore Chains. Abrafarma em números. 2017. Portal ABRAFARMA. Available in https://www.abrafarma.com.br/sobre-1-c1mp6 Accessed: August 6th, 2021.
Adams E, Neves BB, Prola LDT, de Liz MV, Martins LRR, Ramsdorf WA, de Freitas AM (2020) Ecotoxicity and genotoxicity assessment of losartan after UV/H2O2 and UVC/photolysis treatments. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-11420-9
Arrais PSD, Fernandes MEP, da Pizzol TSD, Ramos LR, Mengue SS, Luiza VL, Bertoldi AD (2016) Prevalence of self-medication in Brazil and associated factors. Rev Saude Publica 50(Suppl 2). https://doi.org/10.1590/s1518-8787.2016050006117
Arola K, Hatakka H, Mänttäri M, Kallioinen M (2017) Novel process concept alternatives for improved removal of micropollutants in wastewater treatment. Sep Purif Technol 186:333–341. https://doi.org/10.1016/j.seppur.2017.06.019
Ashfaq M, Li Y, Rehman MSU, Zubair M, Mustafa G, Nazar MF, Sun Q (2019) Occurrence, spatial variation and risk assessment of pharmaceuticals and personal care products in urban wastewater, canal surface water, and their sediments: a case study of Lahore, Pakistan. Science of the Total Environment. doi:https://doi.org/10.1016/j.scitotenv.2019.06.285
Becker RW, Alves Jachstet L, Dallegrave A, Ruiz-Padillo A, Zanella R, Sirtori C (2021) Multi-criteria decision-making techniques associated with (Q)SAR risk assessment for ranking surface water microcontaminants identified using LC-QTOF MS. Sci Total Environ 797:149002. https://doi.org/10.1016/j.scitotenv.2021.149002
Besse J-P, Garric J (2008) Human pharmaceuticals in surface waters. Toxicol Lett 176(2):104–123. https://doi.org/10.1016/j.toxlet.2007.10.012
Biel-Maeso M, Baena-Nogueras RM, Corada-Fernández C, Lara-Martín PA (2018) Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci Total Environ 612:649–659. https://doi.org/10.1016/j.scitotenv.2017.08.2
Biudes JFV, Camargo AFM (2006) Changes in biomass, chemical composition and nutritive value of Spartina alterniflora due to organic pollution in the Itanhaém River Basin (SP, Brazil). Braz J Biol 66:781–789. https://doi.org/10.1590/S1519-69842006000500003
Bouissou-Schurtz C, Houeto P, Guerbet M, Bachelot M, Casellas C, Mauclaire A-C, Masset D (2014) Ecological risk assessment of the presence of pharmaceutical residues in a French national water survey. Regul Toxicol Pharmacol 69(3):296–303. https://doi.org/10.1016/j.yrtph.2014.04.006
Capolupo M, Valbonesi P, Kiwan A, Buratti S, Franzellitti S, Fabbri E (2016) Use of an integrated biomarker-based strategy to evaluate physiological stress responses induced by environmental concentrations of caffeine in the Mediterranean mussel Mytilus galloprovincialis. Sci Total Environ 563-564:538–548. https://doi.org/10.1016/j.scitotenv.2016.04.125
Cavalcanti IFA, Nunes LH, Marengo JA, Gomes JL, Silveira VP, Castellano MS (2017) Projections of precipitation changes in two vulnerable regions of São Paulo State, Brazil. Am J Clim Chang 6:268–293. https://doi.org/10.4236/ajcc.2017.62014
Čelić M, Gros M, Farré M, Barceló D, Petrović M (2019) Pharmaceuticals as chemical markers of wastewater contamination in the vulnerable area of the Ebro Delta (Spain). Sci Total Environ 652:952–963. https://doi.org/10.1016/j.scitotenv.2018.10.2
Cetesb - Companhia Estadual de Tecnologia e Saneamento ambiental (2020) Relatório de qualidade das águas costeiras no estado de São Paulo 2019. Série Relatórios/Agência Ambiental do Estado de São Paulo. ISSN 0103-4103
Clark B, Henry GLH, Mackay D (1995) Fugacity analysis and model of organic chemical fate in a sewage treatment plant. Environ Sci Technol 29(6):1488–1494. https://doi.org/10.1021/es00006a009
CMED - Câmara de Regulação do Mercado de Medicamentos (2019) Anuário Estatístico do Mercado Farmacêutico. ANVISA, Brasília, Brazil. Available in: http://portal.anvisa.gov.br/
Cortez FS, da Souza LS, Guimarães LL, Almeida JE, Pusceddu FH, Maranho LA, Pereira CDS (2018) Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). Sci Total Environ 637-638:1363–1371. https://doi.org/10.1016/j.scitotenv.2018.05.0
Dafouz R, Cáceres N, Rodríguez-Gil JL, Mastroianni N, López de Alda M, Barceló D, Valcárcel Y (2018) Does the presence of caffeine in the marine environment represent an environmental risk? A regional and global study. Sci Total Environ 615:632–642. https://doi.org/10.1016/j.scitotenv.2017.09.155
Daouk S, Chèvre N, Vernaz N, Bonnabry P, Dayer P, Daali Y, Fleury-Souverain S (2015) Prioritization methodology for the monitoring of active pharmaceutical ingredients in hospital effluents. J Environ Manag 160:324–332. https://doi.org/10.1016/j.jenvman.2015.06.037
De Andrade Aragão RB, Semensatto D, Calixto LA, Labuto G (2020) Pharmaceutical market, environmental public policies and water quality: the case of the São Paulo Metropolitan Region. Brazil Cad Saúde Pública 36. https://doi.org/10.1590/0102-311X00192319
Deen J, Von Seidlein L (2019) Paracetamol for dengue fever: no benefit and potential harm? Lancet Glob Health 7(5):e552–e553. https://doi.org/10.1016/s2214-109x(19)30157-3
Di Nica V, Villa S, Finizio A (2016) Toxicity of individual pharmaceuticals and their mixtures to Aliivibrio fischeri: experimental results for single compounds and considerations of their mechanisms of action and potential acute effects on aquatic organisms. Environ Toxicol Chem 36(3):807–814. https://doi.org/10.1002/etc.3568
Dos Santos JC, Faria Junior M, Restini CBA (2012) Potential drug interactions identified in prescriptions to hypertensive patients. Revista Brasileira de Clinica Medica São Paulo 10(4):308–317
Dufresne C, Arfib B, Ducros L, Duffa C, Giner F, Rey V (2020) Karst and urban flood-induced solid discharges in Mediterranean coastal rivers: the case study of Las River (SE France). J Hydrol 125194. https://doi.org/10.1016/j.jhydrol.2020.125194
ECB. (2003) Technical guidance document on risk assessment for existing substances, part II, pp. 108-110
EC (2015) European Commission. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council (notified under document C (2015) 1756). 3 p. 2015
ECHA. (2008) Guidance on information requirements and chemical safety assessment. Chapter R.10: Characterisation of dose [concentration]-response for environment, pp7-29
EMA - European Medicines Agency, Committee for Medicinal Products for Human use (CHMP) (2006) Guideline on the environmental risk assessment of medicinal products for human use. Doc. Ref.: EMEA/CHMP/SWP/4447/00 corr 1, London, UK
Fernández C, González-Doncel M, Pro J, Carbonell G, Tarazona JV (2010) Occurrence of pharmaceutically active compounds in surface waters of the henares-jarama-tajo river system (madrid, spain) and a potential risk characterization. Sci Total Environ 408(3):543–551. https://doi.org/10.1016/j.scitotenv.2009.10.0
Fernández-Rubio J, Rodríguez-Gil JL, Postigo C, Mastroianni N, López de Alda M, Barceló D, Valcárcel Y (2019a) Psychoactive pharmaceuticals and illicit drugs in coastal waters of north-western Spain: environmental exposure and risk assessment. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.02
Ferreira FC, Petrere M (2009) The fish zonation of the Itanhaém River basin in the Atlantic Forest of southeast Brazil. Hydrobiologia 636(1):11–34. https://doi.org/10.1007/s10750-009-9932-4
Fernández-Rubio J, Rodríguez-Gil JL, Postigo C, Mastroianni N, López de Alda M, Barceló D, Valcárcel Y (2019b) Psychoactive pharmaceuticals and illicit drugs in coastal waters of north-western Spain: environmental exposure and risk assessment. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.02
Fontes MK, de Campos BG, Cortez FS, Pusceddu FH, Moreno BB, Maranho LA, Pereira CDS (2019) Seasonal monitoring of cocaine and benzoylecgonine in a subtropical coastal zone (Santos Bay, Brazil). Mar Pollut Bull 149:110545. https://doi.org/10.1016/j.marpolbul.2019.110545
Fontes MK, de Campos BG, Cortez FS, Pusceddu FH, Nobre CR, Moreno BB, Pereira CDS (2020) Mussels get higher: a study on the occurrence of cocaine and benzoylecgonine in seawater, sediment and mussels from a subtropical ecosystem (Santos Bay, Brazil). Sci Total Environ 143808. https://doi.org/10.1016/j.scitotenv.2020.143808
de Freitas LAA, Radis-Baptista G (2021) Pharmaceutical pollution and disposal of expired, unused, and unwanted medicines in the Brazilian context. J Xenobiotics 11(2):61–76. https://doi.org/10.3390/jox11020005
Hanlon JT, Perera S, Newman AB, Thorpe JM, Donohue JM, Simonsick EM (2017) Potential drug-drug and drug-disease interactions in well-functioning community-dwelling older adults. J Clin Pharm Ther 42(2):228–233. https://doi.org/10.1111/jcpt.12502
Hernando MD, Mezcua M, Fernandez-Alba AR, Barcelo D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69(2):334–342. https://doi.org/10.1016/j.talanta.2005.09.037
Galus M, Jeyaranjaan J, Smith E, Li H, Metcalfe C, Wilson JY (2013) Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat Toxicol 132-133:212–222. https://doi.org/10.1016/j.aquatox.2012.12.016
González-Mariño I, Baz-Lomba JA, Alygizakis NA, Andrés-Costa MJ, Bade R, Barron LP, Bodík I (2019) Spatio-temporal assessment of illicit drug use at large scale: evidence from 7 years of international wastewater monitoring. Addiction. https://doi.org/10.1111/add.14767
Hamid N, Junaid M, Wang Y, Pu S-Y, Jia P-P, Pei D-S (2021) Chronic exposure to PPCPs mixture at environmentally relevant concentrations (ERCs) altered carbohydrate and lipid metabolism through gut and liver toxicity in zebrafish. Environ Pollut 273:116494. https://doi.org/10.1016/j.envpol.2021.116494
Ibge – Instituto brasileiro de Geografia e Estatística (2019) Estimativa da população brasileira. Brasil, Rio de Janeiro
Korekar G, Kumar A, Ugale C (2019) Occurrence, fate, persistence, and remediation of caffeine: a review. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-06998-8
Lacorte S, Luis S, Gómez-Canela C, Sala-Comorera T, Courtier A, Roig B, Calas-Blanchard C (2017) Pharmaceuticals released from senior residences: occurrence and risk evaluation. Environ Sci Pollut Res 25(7):6095–6106. https://doi.org/10.1007/s11356-017-9755-1
Lawrence JR, Zhu B, Swerhone GDW, Roy J, Tumber V, Waiser MJ, Korber DR (2012) Molecular and microscopic assessment of the effects of caffeine, acetaminophen, diclofenac, and their mixtures on river biofilm communities. Environ Toxicol Chem 31(3):508–517. https://doi.org/10.1002/etc.1723
Maasz G, Mayer M, Zrinyi Z, Molnar E, Kuzma M, Fodor I, Takács P (2019) Spatiotemporal variations of pharmacologically active compounds in surface waters of a summer holiday destination. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.04.286
Machado KC, Grassi MT, Vidal C, Pescara IC, Jardim WF, Fernandes AN, Severo FJR (2016) A preliminary nationwide survey of the presence of emerging contaminants in drinking and source waters in Brazil. Sci Total Environ 572:138–146. https://doi.org/10.1016/j.scitotenv.2016.07.210
Maranho LA, dos Santos DM, da Fonseca TG, Ortega ADSB, da Silva Sousa L, Pusceddu FH, Pereira CDS (2021) Occurrence and environmental fate of pharmaceuticals, personal care products and illicit drugs (PPCPIDs) in tropical ecosystems. Pharma Marine Coast Environ, 169-193
Mathon B, Choubert J-M, Miege C, Coquery M (2016) A review of the photodegradability and transformation products of 13 pharmaceuticals and pesticides relevant to sewage polishing treatment. Sci Total Environ 551-552:712–724. https://doi.org/10.1016/j.scitotenv.2016.02.0
McNally RJ, Morselli F, Farukh B, Chowienczyk PJ, Faconti L (2019) A review of the prescribing trend of thiazide-type and thiazide like diuretics in hypertension: a UK perspective. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.14109
Mello FV, Cunha SC, Fogaça FHS, Alonso MB, Torres JPM, Fernandes JO (2022) Occurrence of pharmaceuticals in seafood from two Brazilian coastal areas: implication for human risk assessment. Sci Total Environ 803:149744. https://doi.org/10.1016/j.scitotenv.2021.1497
Moermond CT, Janssen MP, de Knecht JA, Montforts MH, Peijnenburg WJ, Zweers PG, Sijm DT (2012) PBT assessment using the revised annex XIII of REACH: a comparison with other regulatory frameworks. Integr Environ Assess Manag 8(2):359–371. https://doi.org/10.1002/ieam.1248
Moreira LB, Leite PRBD, de Dias ML, de Martins CC, de Abessa DMS (2019) Sediment quality assessment as potential tool for the management of tropical estuarine protected areas in SW Atlantic, Brazil. Ecol Indic 101:238–248. https://doi.org/10.1016/j.ecolind.2018.12.052
Moreira LB, Vicente TM, Taniguchi S, Hortellani MA, Sarkis JES, Bícego MC, de Abessa DMS (2017) Assessing legacy contaminants in sediments from marine protected areas of the central coast of São Paulo (Brazil). Braz J Oceanogr 65(4):549–563. https://doi.org/10.1590/s1679-87592017128806504
O’Flynn D, Lawler J, Yusuf A, Parle-McDermott A, Harold D, Mc Cloughlin T, White B (2021) A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic. Anal Methods 13(5):575–594. https://doi.org/10.1039/d0ay02098b
Nunes B, Verde MF, Soares AMVM (2015) Biochemical effects of the pharmaceutical drug paracetamol on Anguilla anguilla. Environ Sci Pollut Res 22(15):11574–11584. https://doi.org/10.1007/s11356-015-4329-6
Pereira CDS, Maranho LA, Cortez FS, Pusceddu FH, Santos AR, Ribeiro DA, Cesar A, Guimarães LL (2016) Occurrence of pharmaceuticals and cocaine in a Brazilian coastal zone. Sci Total Environ 548-549:148–154. https://doi.org/10.1016/j.scitotenv.2016.01.051
Pereira AL, de Vasconcelos Barros RT, Pereira SR (2017) Pharmacy pollution and household waste medicine (HWM): how reverse logistics is environmentally important to Brazil. Environ Sci Pollut Res 24(31):24061–24075. https://doi.org/10.1007/s11356-017-0097-9
Pires A, Almeida Â, Correia J, Calisto V, Schneider RJ, Esteves VI, Freitas R (2016) Long-term exposure to caffeine and carbamazepine: impacts on the regenerative capacity of the polychaete Diopatra neapolitana. Chemosphere 146:565–573. https://doi.org/10.1016/j.chemosphere.2015.12.035
Pusceddu FH, Guimarães MM, Lopes LO, Souza LS, Cortez FS, Pereira CDS, Cesar A (2022) Biological effects of the antihypertensive losartan under different ocean acidification scenarios. Environ Pollut 292:118329
Quadra GR, Paranaíba JR, Vilas-Boas J, Roland F, Amado AM, Barros N, Cardoso SJ (2019) A global trend of caffeine consumption over time and related-environmental impacts. Environ Pollut 113343. https://doi.org/10.1016/j.envpol.2019.113343
Ramos AS, Correia AT, Antunes SC, Gonçalves F, Nunes B (2014) Effect of acetaminophen exposure in Oncorhynchus mykiss gills and liver: detoxification mechanisms, oxidative defence system and peroxidative damage. Environ Toxicol Pharmacol 37(3):1221–1228. https://doi.org/10.1016/j.etap.2014.04.005
Reque R, Carneiro RD, Yamamoto FY, Ramsdorf WA, Martins LR, Guiloski IC, de Freitas AM (2021) Ecotoxicity of losartan potassium in aquatic organisms of different trophic levels. Environ Toxicol Pharmacol 87:103727. https://doi.org/10.1016/j.etap.2021.103727
Robles-Molina J, Gilbert-López B, García-Reyes JF, Molina-Díaz A (2014) Monitoring of selected priority and emerging contaminants in the Guadalquivir River and other related surface waters in the province of Jaén, South East Spain. Sci Total Environ 479-480:247–257. https://doi.org/10.1016/j.scitotenv.2014.01.121
Ribeiro TS, Carvalho DP, Guimarães MT, Campina NN, Lobarinhas MR, Lopes ALJ, Braga ALF (2016) Prevalence of hypertension and its associated factors in contaminated areas of the Santos-São Vicente Estuarine region and Bertioga, Brazil: 2006-2009. Environ Sci Pollut Res 23(19):19387–19396. https://doi.org/10.1007/s11356-016-7138-7
Roveri V, Barrella V, Ramires M (2012) Instruments of Law 9985/00: a discussion of its effectiveness in environmental management Mosaic Ecological Station Juréia / Itatins, from Law 12.406/06. Law and Social. Science 1(1):ISSN: 2317-1308
Roveri V, Guimarães LL, Toma, Correia AT (2020a) Occurrence and ecological risk assessment of pharmaceuticals and cocaine in a beach area of Guarujá, São Paulo State, Brazil, under the influence of urban surface runoff. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-10316-y
Roveri V, Guimarães LL, Correia AT (2020b) Spatial and temporal evaluation of the urban runoff water flowing into recreational areas of Guarujá, São Paulo State, Brazil. Intl J River Basin Manag 1–0. https://doi.org/10.1080/15715124.2020.1776304
Roveri V, Guimarães LL, Toma W, Correia AT (2021) Occurrence and ecological risk assessment of pharmaceuticals and cocaine in the urban drainage channels of Santos beaches (São Paulo, Brazil): a neglected, but sensitive issue. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15249-8
São Paulo (2008) State Decree No. 53.528.Decreto de criação do Mosaico de Áreas Marinhas Protegidas do Litoral Paulista. N° 53.528, de 8 de outubro de 2008. D.O.E.
Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello C, Zoccoli A, Capuano A (2020) Current pharmacological treatments for COVID-19: what’s next? Br J Pharmacol. https://doi.org/10.1111/bph.15072
Shihomatzu, H. M., 2015. Desenvolvimento e Validação de Metodologia SPE-LC-MS/MS para determinação de Fármacos e Droga de Abuso nas Águas da Represa Guarapiranga, São Paulo/SP, Brasil. IPEN/USP. https://doi.org/10.11606/T.85.2015.tde-28042015-095207
Secoli S-R, Figueras A, Lebrão ML, de Lima FD, Santos JLF (2010) Risk of potential drug-drug interactions among Brazilian elderly. Drugs Aging 27(9):759–770. https://doi.org/10.2165/11538460-000000000-00000
SMA/CPLA – Secretaria de Meio Ambiente do Estado de São Paulo/Coordenação de Planejamento Ambiental (2018) Zona Costeira Paulista: Relatório de Qualidade Ambiental. Org. Organizadores Nádia Gilma Beserra de Lima e Tatiana Camolez Morales Ferreira. SMA/CPLA, São Paulo. Brasil
SMA/CPLEA – Secretaria do Meio Ambiente do Estado de São Paulo/Coordenadoria de Planejamento e Educação Ambiental (2016) Zoneamento Ecológico - Econômico – Baixada Santista, São Paulo, Brasil, 55 p
UNODC - United Nations Office on Drugs and Crime (2020) World drug report, 2020. Booklet 1. Executive summary. Available at: https://wdr.unodc.org/wdr2020/en/exsum.html
USEPA - United States Environmental Protection Agency (2007) Method 1694: pharmaceuticals and personal care products in water, soil sediment, and biosolids by HPLC/MS/MS. Washington
USEPA - United States Environmental Protection Agency (2017a) Ecological Structure-Activity Relationship Model (ECOSAR) class program. MS-Windows version 2.0. https://www.epa.gov/tsca732screening-tools/ecological-structure-activity-relationships-ecosarcpredictive-model
USEPA - United States Environmental Protection Agency (2017b) Estimation Program Interface Suite (EPI Suite™). Class program. MS-Windows version 4.11. https://www.epa.gov/tsca-screening-tools/download-epi-suitetm-estimation-program-interface-v411
USEPA - United States Environmental Protection Agency, 2019. ECOTOX user guide: Ecotoxicology Database system, version 4.0. http://www.epa.gov/ecotox/.
Vazquez-Roig P, Andreu V, Blasco C, Picó Y (2012) Risk assessment on the presence of pharmaceuticals in sediments, soils and waters of the Pego–Oliva Marshlands (Valencia, eastern Spain). Sci Total Environ 440:24–32. https://doi.org/10.1016/j.scitotenv.2012.08.036
Yamamoto NS, Dias C, Pereira S, Cortez FS, Pusceddu H, Santos AR, Guimaraes LL (2012) Avaliação dos efeitos biológicos do farmaco Losartan em microcrustaceos Daphnia similis e Ceriodaphnia dubia (Crustacea, Cladocera). Unisanta Bioscience 1:49–53
Zhou SB, Paolo CD, Wu XD, Shao Y, Seiler TB, Hollert H (2019) Optimization of screening-level risk assessment and priority selection of emerging pollutants – the case of pharmaceuticals in European surface waters. Environ Int 128:1–10
Acknowledgements
Authors want to acknowledge Daniel Temponi Lebre from the Centro de Espectrometria de Massa Aplicada - Instituto de Pesquisas Energéticas e Nucleares (CEMSA, IPEN, São Paulo, Brazil) for the technical support regarding the LC-MS/MS analyses. Thanks also to Professor Ítalo Braga de Castro, from Instituto do Mar, Universidade Federal de São Paulo (IMAR-UNIFESP, São Paulo, Brazil), for the technical support with the manifold.
Availability of data and materials
Not applicable.
Funding
Correia AT was supported by national funds through the Foundation for Science and Technology (FCT) within the scope of UIDB/04423/2020 and UIDP/04423/2020.
Author information
Authors and Affiliations
Contributions
VR: conceptualization, data curation, formal analysis, methodology and writing of the original draft. LLG: review and editing. WT: review and editing. ATC: writing as well as review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Roland Peter Kallenborn
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 605 kb)
Rights and permissions
About this article
Cite this article
Roveri, V., Guimarães, L.L., Toma, W. et al. Occurrence, ecological risk assessment and prioritization of pharmaceuticals and abuse drugs in estuarine waters along the São Paulo coast, Brazil. Environ Sci Pollut Res 29, 89712–89726 (2022). https://doi.org/10.1007/s11356-022-21945-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21945-w