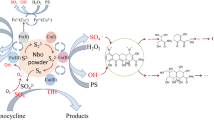
Abstract
Oxidation–reduction-absorption based on sulfite is a promising process for simultaneous removal of NOx and SO2. However, excessive oxidation of sulfite and competitive absorption between NOx and SO2 limit its application. A matching strategy between antioxidants and alkaline agents has been proposed to solve these problems and enhance the absorption process. The comparison results of inhibitors showed that hydroquinone exhibited long-term high-efficiency inhibition of S(IV) (SO32-/HSO3-) oxidation. The comparison of alkaline agents showed that the Na2SO3 solution with heterogeneous mixture of MgO and hydroquinone exhibited better absorption performance than that with other combinations. The absorption amounts of NOx in 0.15 mol/L Na2SO3 50 mL solution added 0.1% hydroquinone (HQ) with 0.09 mol/L MgO were 2.24 mmol, which improved 5 times than that without additives. In addition, studies on the influence of pH showed that the pH of MgO mixture could be stabilized at 9–10 for a long time, while the pH of Na2CO3 mixture decreased faster. Further studies suggested that the hydration of MgO resulted in the solution with MgO keeping high pH. This is also the main reason why the combination of MgO and hydroquinone is superior to the combination of Na2CO3 and hydroquinone in desulfurization and denitration performance.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Adewuyi YG, Khan MA (2016) Nitric oxide removal from flue gas by combined persulfate and ferrous–EDTA solutions: Effects of persulfate and EDTA concentrations, temperature, pH and SO2. Chem Eng J 304:793–807. https://doi.org/10.1016/j.cej.2016.06.071
Busca G, Lietti L, Ramis G, Berti F (1998) Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: a review. Appl Catal B-Environ 18:1–36. https://doi.org/10.1016/S0926-3373(98)00040-X
Chen M, Deng X, He F (2016) Removal of SO2 from flue gas using basic aluminum sulfate solution with the byproduct oxidation inhibition by ethylene glycol. Energ Fuel 30:1183–1191. https://doi.org/10.1021/acs.energyfuels.5b02411
Dhayal Y, Chandel C, Gupta K (2014) The influence of hydroxyl volatile organic compounds on the oxidation of aqueous sulfur dioxide by oxygen. Environ Sci Pollut R 21:7805–7817. https://doi.org/10.1007/s11356-014-2661-x
Fang W (2021) Current situation and prospect of denitration technology in nonferrous smelting industry. IOP Conf Ser Earth Environ Sci 781:052035. https://doi.org/10.1088/1755-1315/781/5/052035
Guo L, Han C, Zhang S, Zhong Q, Ding J, Zhang B, Zeng Y (2018) Enhancement effects of O2− and OH radicals on NOx removal in the presence of SO2 by using an O3/H2O2 AOP system with inadequate O3 (O3/NO molar ratio= 0.5). Fuel 233:769–777. https://doi.org/10.1016/j.fuel.2018.06.099
Guo Q, He Y, Sun T, Wang Y, Jia J (2014) Simultaneous removal of NOx and SO2 from flue gas using combined Na2SO3 assisted electrochemical reduction and direct electrochemical reduction. J Hazard Mater 276:371–376. https://doi.org/10.1016/j.jhazmat.2014.05.058
Hao R, Yang S, Zhao Y, Zhang Y, Yuan B, Mao X (2017a) Follow-up research of ultraviolet catalyzing vaporized H2O2 for simultaneous removal of SO2 and NO: Absorption of NO2 and NO by Na-based WFGD byproduct (Na2SO3). Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2017.02.021
Hao R, Zhang Y, Wang Z, Li Y, Yuan B, Mao X, Zhao Y (2017b) An advanced wet method for simultaneous removal of SO2 and NO from coal-fired flue gas by utilizing a complex absorbent. Chem Eng J 307:562–571. https://doi.org/10.1016/j.cej.2016.08.103
Hao R, Mao Y, Mao X, Wang Z, Gong Y, Zhang Z, Zhao Y (2019) Cooperative removal of SO2 and NO by using a method of UV-heat/H2O2 oxidation combined with NH4OH-(NH4)2SO3 dual-area absorption. Chem Eng J 365:282–290. https://doi.org/10.1016/j.cej.2019.02.059
Jerzy B, Manfred J (1995) Gas-liquid equilibria in the system SO2-aqueous solutions of NaHSO3/Na2SO3/Na2SO4. Chem Eng Sci 50:3067–3075. https://doi.org/10.1016/0009-2509(95)00144-T
Johns F, Hahm P, Thome D, Bretthauer E, Black S, Smith A, Costa C, Beiriger J, Failor R, Marsh K (1988) Rate constant for the reaction of NO2 with sulfur(IV) over the pH range 5.3-13. Environ Sci Technol 22:586–589. https://doi.org/10.1021/es00170a018
Kang MS, Shin J, Yu TU, Hwang J (2020) Simultaneous removal of gaseous NOx and SO2 by gas-phase oxidation with ozone and wet scrubbing with sodium hydroxide. Chem Eng J 381:122601. https://doi.org/10.1016/j.cej.2019.122601
Koide K, Sato H, Iwamoto S (1983) Gas holdup and volumetric liquid-phase mass transfer coefficient in bubble column with draught tube and with gas dispersion into annulus. J Chem Eng Jpn 16(5):407–413. https://doi.org/10.1252/jcej.16.407
Li B, Wu H, Liu X, Zhu T, Zhao X (2020) Simultaneous removal of SO2 and NO using a novel method with red mud as absorbent combined with O3 oxidation. J Hazard Mater 392:122270. https://doi.org/10.1016/j.jhazmat.2020.122270
Li S, Xu H, Huang W, Sun Y, Yan N (2021) NOx absorption enhancement and sulfite oxidation inhibition via a match strategy in Na2SO3 solution from a wet flue gas denitration system. ACS ES&T Engineering 1:100–109. https://doi.org/10.1021/acsestengg.0c00075
Li S, Huang W, Xu H, Liu K, Wang JN, Sun Y, Qu Z, Yan N (2022) Enhanced simultaneous absorption of NOx and SO2 in oxidation-reduction-absorption process with a compounded system based on Na2SO3. J Environ Sci 111:1–10. https://doi.org/10.1016/j.jes.2021.03.003
Lim PK, Huss AJ, Eckert CA (1982) Oxidation of aqueous sulfur dioxide. 3. The effects of chelating agents and phenolic antioxidants. J Phys Chem 86:4233–4237. https://doi.org/10.1021/j100218a029
Liu W, Zhou Y, Hua Y, Peng B, Qu Z (2019) A sulfur-resistant CuS-modified active coke for mercury removal from municipal solid waste incineration flue gas. Environ Sci Pollut R 26:24831–24839. https://doi.org/10.1007/s11356-019-05645-6
Ma Y, Yuan D, Zhang X, Qu Z, Huang W (2020) Inhibiting oxidation and enhancing absorption characteristics of sodium sulfite for SO2 removal from the non-ferrous smelting flue gas. Environ Eng Res 26:200043. https://doi.org/10.4491/eer.2020.043
Meena VK, Dhayal Y, Rathore DS, Chandel CS, Gupta K (2017) Inhibition of atmospheric aqueous phase autoxidation of sulphur dioxide by volatile organic compounds: mono-, di-and tri-substituted benzenes and benzoic acids. Prog React Kinet Mech 42:111–125. https://doi.org/10.3184/146867817X14806858832108
Merwe E, Strydom CA (2006) Hydration of medium reactive magnesium oxide using hydration agents. J Therm Anal Calorim 84:467–471. https://doi.org/10.1007/s10973-005-7291-6
Nash T (1979) The effect of nitrogen dioxide and of some transition metals on the oxidation of dilute bisulphite solutions. Atmos Environ 13:1149–1154. https://doi.org/10.1016/0004-6981(79)90038-6
Obradovi BM, Sretenovi GB, Kuraica MM (2011) A dual-use of DBD plasma for simultaneous NOx and SO2 removal from coal-combustion flue gas. J Hazard Mater 185:1280–1286. https://doi.org/10.1016/j.jhazmat.2010.10.043
Price D, Birnbaum R, Batiuk R, Mccullough M, Smith R (1997) Nitrogen oxides: impacts on public health and the environment. Energy Plan Policy 82:501–524
Qiangwei L, Lidong W, Yi Z, Yongliang M, Shuai C, Shuang L, Peiyao X, Jiming H (2014) Oxidation rate of magnesium sulfite catalyzed by cobalt ions. Environ Sci Technol 48:4145–4152. https://doi.org/10.1021/es404872w
del Valle-Zermeo R, Formosa J, Chimenos JM (2015) Wet flue gas desulfurization using alkaline agents. Rev Chem Eng 31:303–327. https://doi.org/10.1515/revce-2015-0002
Sapkota VNA, Fine NA, Rochelle GT (2015) NO2-catalyzed sulfite oxidation. Ind Eng Chem Res 54:4815–4822. https://doi.org/10.1021/ie504767w
Shen Z, Chen X, Tong M, Guo S, Ni Lu (2013) Studies on magnesium-based wet flue gas desulfurization process with oxidation inhibition of the byproduct. Fuel 105:578–584. https://doi.org/10.1016/j.fuel.2012.07.050
Shuang Z, Shiliang L, Xiaoyun H, Fangyan C, Xue W, Shikui D, Robert B (2018) Temporal dynamics of SO2 and NOx pollution and contributions of driving forces in urban areas in China. Environ Pollut 242:239–248. https://doi.org/10.1016/j.envpol.2018.06.085
Su C, Ran X, Hu J, Shao C (2013) Photocatalytic process of simultaneous desulfurization and denitrifkation of flue gas by TiO2-polyacrylonitrile nanofibers. Environ Sci Technol 47:11562–11568. https://doi.org/10.1021/es4025595
Y Sun E Zwolińska AG Chmielewski 2016 Abatement technologies for high concentrations of NOx and SO2 removal from exhaust gases: a review Crit Rev Environ SciTechnol 119–142 https://doi.org/10.1080/10643389.2015.1063334
Sun Y, Hong X, Zhu T, Guo X, Xie D (2017) The chemical behaviors of nitrogen dioxide absorption in sulfite solution. Appl Sci 7:377. https://doi.org/10.3390/app7040377
Tang N, Liu Y, Wang H, Xiao L, Wu Z (2010) Enhanced absorption process of NO2 in CaSO3 slurry by the addition of MgSO4. Chem Eng J 160:145–149. https://doi.org/10.1016/j.cej.2010.03.022
Wang L, Ma Y, Zhang W, Li Q, Zhao Y, Zhang Z (2013) Macrokinetics of magnesium sulfite oxidation inhibited by ascorbic acid. J Hazard Mater 258:61–69. https://doi.org/10.1016/j.jhazmat.2013.04.018
Wang X, Liu Y, Wu Z (2020) The poisoning mechanisms of different zinc species on a ceria-based NH3-SCR catalyst and the co-effects of zinc and gas-phase sulfur/chlorine species. J Colloid Interface Ence 566:153–162. https://doi.org/10.1016/j.jcis.2020.01.058
Wilkinson PM, Doldersum B, Cramers PH, Van Dierendonck LL (1993) The kinetics of uncatalyzed sodium sulfite oxidation. Chem Eng Sci 48:933–941. https://doi.org/10.1016/0009-2509(93)80331-J
Wu Q, Sun C, Wang H, Wang T, Wang Y, Wu Z (2018) The role and mechanism of triethanolamine in simultaneous absorption of NOx and SO2 by magnesia slurry combined with ozone gas-phase oxidation. Chem Eng J 341:157–163. https://doi.org/10.1016/j.cej.2018.01.150
Wu Z, Wang H, Liu Y, Jiang B, Sheng Z (2008) Study of a photocatalytic oxidation and wet absorption combined process for removal of nitrogen oxides. Chem Eng J 144:221–226. https://doi.org/10.1016/j.cej.2008.01.025
Yuan QA, Chi WB, Xin SA, Ym A, Xin SA, Fei WA, Kai LA, Ping NA (2021) Defects on activated carbon determine the dispersion of active components and thus the simultaneous removal efficiency of SO2, NOx and Hg0. Fuel 293:120391. https://doi.org/10.1016/j.fuel.2021.120391
Zhang J, Zhang R, Chen X, Tong M, Lu J (2014) Simultaneous removal of NO and SO2 from flue gas by ozone oxidation and NaOH absorption. Ind Eng Chem Res 53:6450–6456. https://doi.org/10.1021/ie403423p
Funding
This work was financially supported by the National Natural Science Foundation of China (No.21976118) and the Startup Fund for Youngman Research at SJTU (No. 19X100040083).
Author information
Authors and Affiliations
Contributions
Wenjun Huang and Sichao Li: conceptualization, methodology, data curation, writing-original draft. Haomiao Xu, Hongbin Wang, and Peng Cui: writing-review and editing, supervision. Can Cheng, Zan Qu, and Naiqiang Yan: supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11356_2022_20748_MOESM1_ESM.docx
Supplementary file1 (DOCX 197 kb) The Schematic diagram of absorption experimental setup was shown in Fig. S1. The oxidation-reduction-absorption (O-r-A) process was shown in Fig. S2. The effect of additive amount on the increase of NOx absorption amount by unit additives amount in Fig. S3. The dependence of Mg2+ concentration and pH value with reaction time was shown in Figure S4. The reaction agents costs of the removal SO2 and NOx were listed in Table S1.
Rights and permissions
About this article
Cite this article
Huang, W., Li, S., Wang, H. et al. Buffer effect of MgO on Na2SO3 to stabilize S(IV) for the enhancement in simultaneous absorption of NOx and SO2 from non-ferrous smelting gas. Environ Sci Pollut Res 29, 71721–71730 (2022). https://doi.org/10.1007/s11356-022-20748-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20748-3