Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease (ND) that represents the principal cause of dementia. Effective treatment is still lacking. Without prevention, Alzheimer’s disease (AD) incidence is expected to triple within 30 years. The risk increases in highly polluted areas and is positively linked to chronic aluminum (Al) exposure. Canonical Wingless-Int (Wnt)/β-catenin pathway has been found to play a considerable role in ND pathogenesis. Resins of Boswellia serrata (frankincense) have been used traditionally for their psychoactive activity, in addition to their memory-boosting effects. Boswellic acids (BA) are pentacyclic triterpenes. They have antioxidant, anti-inflammatory, antinociceptive, and immunomodulatory activities. This study aimed to elucidate the role of the Wnt/β-catenin pathway in BA protective activity against aluminum-induced Alzheimer’s disease. For 6 weeks, rats were treated daily with AlCl3 (100 mg/kg/i.p.) either alone or with BA (125 or 250 mg/kg PO). Results indicated that BA significantly improved learning and memory impairments induced by AlCl3 treatment. Moreover, BA treatment significantly decreased acetylcholinesterase levels and reduced amyloid-beta (Aβ) expression. In addition, BA ameliorated the increased expression of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), inhibited lipid peroxidation, and increased total antioxidants in the brain. Indeed, BA significantly suppressed AlCl3-induced decrease of brain-derived neurotrophic factor, pGSK-3β (Ser 9), and β-catenin. BA (250 mg/kg) showed a significant protective effect compared to a lower dose. The results conclude that BA administration modulated the expression of Wnt/β-catenin pathway-related parameters, contributing to BA’s role against Al-induced Alzheimer’s disease.
Graphical abstract
Effect of Boswellic acids on AlCl3-induced neurodegenerative changes. ChE cholinesterase, Ach acetylcholine, BDNF brain-derived neurotrophic factor, IL-1β interleukin-1β, TNF-α tumor necrosis factor-α

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of neurodegenerative diseases (NDs) is rapidly growing around the world. With the increasing age of the population, these disorders parallelly increase. Alzheimer’s disease (AD) is a ND characterized by behavioral and memory defects, accompanied by functional and cognitive impairments. AD may be considered the most offending cause of dementia in the elderly. Pathological hallmarks of AD include intracellular neurofibrillary tangles aggregated by hyperphosphorylated tau, in addition to extracellular senile plaques of beta-amyloid (Aβ) protein (Peden and Ironside 2012). Several studies have displayed different mechanisms for AD pathogenesis, including oxidative stress, amyloidogenesis theory, cholinergic dysfunction, and neuroinflammation. Although the argument, neuroinflammation and oxidative stress have been established to be the critical pathological marks of AD (Magalingam et al. 2018). AD has been linked to environmental pollution, with several pollutants implicated, such as carbon monoxide, ozone, and particulate matter (Fu and Yung 2020). Aluminum (Al) toxicity has ample evidence of being linked to AD (Colomina and Peris-Sampedro 2017).
The advancement of recognizing the molecular pathological mechanisms underlying AD is mandatory for evolving new therapeutic strategies that help prevent or halt disease progression. Increasing evidence suggests that dysregulation of the canonical Wingless-Int (Wnt)/β-catenin pathway could be embroiled in the NDs pathogenesis. Downregulation of the Wnt/β-catenin signaling cascade has been linked with AD onset and progression and synaptic stability (Jia et al. 2019b). Glycogen synthase kinase 3β (GSK-3β) is a crucial regulator of the Wnt canonical pathway. Activation of this pathway leads to the inhibition of GSK-3β activity and an increase of β-catenin activity. β-catenin is a transcriptional molecule that migrates to the nucleus and stimulates the transcription of target genes. On the contrary, if the Wnt canonical pathway is switched off, GSK-3β activity increases and stimulates β-catenin degradation. Remarkably, GSK-3β/β-catenin has been implicated in neuronal survival, neurodegeneration, and memory integration (Libro et al. 2016). Wnt/β-catenin signaling is critically associated with oxidative stress in AD (Xian et al. 2016; Libro et al. 2016; Vallée et al. 2017; Wang et al. 2019). Furthermore, GSK-3β/Wnt signaling was found to play a role in neurodegeneration in AD through the induction of inflammatory and apoptotic pathways. In addition, Wnt/β-catenin signaling regulates the expression of brain-derived neurotrophic factor (BDNF). BDNF is a vital neurotrophin that governs neuronal cells’ growth, survival, and differentiation. It also modulates cognitive functions and hinders neuroinflammation (Yang et al. 2016).
The application of traditionally used natural products to prevent and treat diseases, especially chronic diseases, has attracted much attention because of their relative safety and scientifically proven activity. Pentacyclic triterpenes are a class of compounds with multiple biological activities, among them Boswellic acids (BAs). BAs are the main constituents separated from the gum resin of Boswellia serrata (recognized as Frankincense or olibanum), such as β-boswellic acid, 3-acetyl-α-boswellic acid, 11-keto-β-boswellic acid, and acetyl-11-keto-β-boswellic acid (AKBA). The Boswellia species (frankincense) resins are well known worldwide. They have traditionally been used in folk medicine in India, China, and by Arabs; furthermore, they are used in Europe for religious rituals (Efferth and Oesch 2020). They were used in folk medicine to treat wounds and inflammatory diseases and their psychoactive effects (Byler and Setzer 2018). Boswellia resin extract suppresses the expression of proinflammatory mediators. In addition, it has antioxidant, anti-nociceptive, and immunomodulatory activities owing to its ability to target different signaling pathways, enzymes, transcription factors, as well as kinases (Al-Harrasi et al. 2019).
Boswellia resin was also utilized to boost the memory and learning activity (Hosseini et al. 2010; Jalili et al. 2014; Majdinasab et al. 2016; Ebrahimpour et al. 2017). Recently, Byler et al. concluded the potential activity of Boswellia resin in Alzheimer’s disease using a docking study, through its effect on acetylcholinesterase (AChE) (Byler and Setzer 2018). In addition, the anti-inflammatory and antiapoptotic properties of Boswellia resin make it a promising target against neuroinflammation, which characterizes NDs (Sayed and El Sayed 2016; Sayed et al. 2018). So, the current study hypothesized that BAs might have a neuroprotective activity against AlCl3-induced AD symptoms. Their effect is partially mediated via regulating the key masters of the Wnt/β-catenin pathway.
Materials and methods
Drugs and chemicals
AlCl3.6H2O was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). It was freshly dissolved in saline, and intraperitoneally (i.p.) injected. Capsules containing standardized B. serrata gum extract (65% BAs equivalent to 292.5 mg) were purchased from GNC Herbal Plus® (Pittsburgh, PA, USA) and dissolved in distilled water. All other chemicals were of the highest analytical grade.
Animals
Forty adult male Sprague Dawley rats (180–200 g) were obtained from the Nile Co. for Pharmaceuticals and Chemical Industries, Cairo, Egypt. Animals were maintained in groups of five per cage in the animal facility of the Faculty of Pharmacy (Girls), Al-Azhar University, housed in a conditioned atmosphere at 25 ± 2 °C. They were kept on standard diet pellets (El-Nasr, Cairo, Egypt) and tap water ad libitum. The experiment was carried out by ethical procedures and policies approved by the Ethics Committee of the Faculty of Pharmacy (Girls), Al-Azhar University (approval no.137).
Experimental design
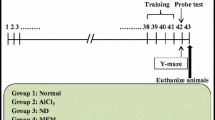
Forty rats were arbitrarily split into four groups (ten animals per group). Group 1 (control group): rats were injected with saline (1 ml/kg, i.p.) and received distilled water orally (1 ml/kg, p.o.); group 2 (AD group): rats were daily injected with AlCl3.6H2O (100 mg/kg, i.p.) (Mohamed et al. 2021); groups 3 and 4 were treated with daily i.p. doses of AlCl3.6H2O and BAs (125 ml/kg or 250 mg/kg, orally) respectively (Ameen et al. 2017). All treatments were given daily for 6 weeks. Then, the behavioral tests were conducted after administering the last doses. Twenty-four hours after the behavioral tests, animals were euthanized, brains were insulated, and the hippocampus was separated from each brain, immediately rinsed in ice-cold normal saline (0.9% w/v), and finally homogenized in 0.1 M phosphate buffer (pH 7.4, the final concentration of 10% w/v) for further biochemical analysis.
Methods
Behavioral testing
Two behavioral tests were used to assess different behavioral changes in rats. The open-field test (OFT) is a mild stressful condition helpful in detecting changes in exploratory behavior and emotionality (Cunha and Masur 1978). The Morris water maze (MWM) test represents a standard test of memory, and spatial learning in rodents, as Morris (1984) described (Morris 1984).
Protein estimation
According to the Bradford technique, the protein content was measured in the hippocampus homogenates (Bradford 1976) using standard bovine serum albumin.
Enzyme-linked immunosorbent assays
Commercially available enzyme-linked immunosorbent assays (ELISA) kits were used to define the levels of hippocampal AChE and amyloid beta-peptide 1–42 (Aβ1-42) (MyBioSource. Inc., USA, catalog no. MBS725468 and MBS726579, respectively), malondialdehyde (MDA) (lifeSpan Biosciences, Inc., USA; catalogue no. LS-F28018), superoxide dismutase (SOD), and total antioxidant capacity (TAC) (MyBioSource. Inc., USA, catalog no. MBS036924 and MBS733414_48T, respectively). ELISA kits from Cusabio Biotech Co., China, were used to measure the levels of IL-1β (catalog no. CSB-E08055r), tumor necrosis factor-alpha (TNF-α) (catalogue no. CSB-E11987r), BDNF (Boster Biological Technology Co., LTD, catalogue no. EK0308), and Beta-catenin (MyBioSource. Inc., USA, catalog no. MBS720420). In addition, pGSK-3β (Ser9) (RayBiotech, Inc., catalog no. PEL-GSK3b-S9-T) was assessed. All assays exactly followed the manufacturer’s instructions.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Data were analyzed using a one-way analysis of variance (ANOVA). Tukey's multiple comparison test was used to assess differences between means. A significant difference was considered at the level of P < 0.05. Statistical analyses and plotting were performed using GraphPad Prism (ISI®, USA) software (version 5).
Results
Determination of behavioral changes
Behavioral results of OFT for different groups are shown in Fig. 1A, B, C, and D. AlCl3 injection significantly reduced the exploratory activity, as evidenced by the decrease in the ambulation frequency by 56.6% and the rearing frequency by 67.6% compared to the control group. Nevertheless, as a manifestation of emotionality, self-grooming and the number of pellets significantly increased after AlCl3 treatment to 160% and 328.6%, respectively, compared to the control rats. AlCl3-induced exploratory and emotional dysregulations improved considerably upon treating animals with either BAs (125 mg/kg) or BAs (250 mg/kg).
Effect of Boswellic acids on Alzheimer’s-induced behavioral alterations in the open-field test. A Ambulation frequency, B rearing frequency, C grooming frequency, D defecation. Data are mean ± SD (n = 8). a, b, or c: significantly different compared to the control, AD group, or BAs (125 mg/kg) group respectively, P < 0.05 using ANOVA followed by Tukey’s as post hoc test. AD Alzheimer’s group (AlCl3 (100 mg/kg)), BAs Boswellic acids
As reported in Fig. 2A, AlCl3 administration caused spatial learning and memory disturbance. The efficiency of the learning ability in animals treated with AlCl3 was diminished, evidenced by a significant increase in escape latency from day 1 to day 4 of training by approximately 80%, 74.7%, 89.9%, and 132.6%, respectively, as compared to control values. However, concurrent treatment of animals with either AlCl3 + BAs (125 mg/kg) or AlCl3 + BAs (250 mg/kg) significantly enhanced learning ability. Rats treated with AlCl3 showed a significant decrease in the time spent in the target quadrant, reflecting memory deficits by approximately 51.7% compared to the control group. However, the AlCl3 + BAs (125 mg/kg) or AlCl3 + BAs (250 mg/kg) group modulate the decreased time spent where they were increased by 53.5% and 102.6%, respectively, compared to those theAlCl3-treated group. The higher dose of BAs produced a better enhancement in memory function (Fig. 2B).
Effect of Boswellic acids on Alzheimer’s-induced behavioral alterations in the Morris Water Maze test. A Escape latency, B time spent in the target quadrant. Data are mean ± SD (n = 8). a, b, or c: significantly different compared to the control, AD group, or BAs (125 mg/kg) group respectively, P < 0.05 using ANOVA followed by Tukey’s as post hoc test. AD Alzheimer’s group (AlCl3 (100 mg/kg)), BAs Boswellic acids
Assessment of AChE and Aβ1-42
Figure 3A showed that the AChE level was markedly elevated in the AlCl3-treated group reaching 717.8% compared to the control group. Concurrent treatment of animals with either AlCl3 + BAs (125 mg/kg) or AlCl3 + BAs (250 mg/kg) significantly decreased AChE levels by 32.6% and 48.1% respectively as compared to animals treated with AlCl3 alone.
Effect of Boswellic acids on Alzheimer’s-induced alterations in the hippocampal acetylcholinesterase and amyloid-beta peptides content. A acetylcholinesterase, B amyloid-beta peptides. Data are mean ± SD (n = 6). a, b, or c: significantly different compared to the control, AD group, or BAs (125 mg/kg) group respectively, P < 0.05 using ANOVA followed by Tukey’s as post hoc test. AD: Alzheimer’s group (AlCl3 (100 mg/kg)); BAs: Boswellic acids
The level of Aβ1-42 was significantly increased in the AlCl3-treated group by 362.5% compared to the control values. However, AlCl3 + BAs (125 mg/kg) or AlCl3 + BAs (250 mg/kg) significantly decreased Aβ1-42 levels when compared to AlCl3-treated group (Fig. 3B).
Estimation of markers of oxidative stress and inflammation
It was found that AlCl3 injection caused an elevation in lipid peroxide formation (measured as MDA contents) that reached 955.2% as compared to the control group. However, BAs significantly decreased MDA in their two doses compared to the AlCl3-treated group. Levels of SOD and TAC of AlCl3-treated rats were significantly reduced by about 86% and 84%, respectively, compared to the control values. Animals treated with either dose of BAs exhibited a significant enhancement in SOD and TAC compared to AlCl3-treated animals (Table 1).
Also, Table 1 displays hippocampal levels of TNF-α and IL-1β in the different treatment groups. AlCl3 showed marked increases in both TNF-α and IL-1β levels by 737.1% and 492%, respectively, compared to the control value. On the other hand, AlCl3 + BAs (125 mg/kg) or AlCl3 + BAs (250 mg/kg) groups modulated the increases in TNF-α and IL-1β tissue levels where they were decreased by (41.1%, 59.8%) and (38.4%, 54.8%) respectively as compared to AlCl3-treated group.
Determination of the hippocampal levels of BDNF, pGSK-3β (Ser 9), and β-catenin
To fulfill the underlying mechanisms incorporated in the neuroprotective effect of BAs, BDNF/GSK-3β/β-catenin axis was evaluated. Treatment with AlCl3 intensely reduced BDNF protein levels by 88.3% compared to the control levels. AlCl3-treated rats simultaneously administered either BAs (125 mg/kg) or BAs (250 mg/kg) showed a significant rise in BDNF levels by 183.1% and 302.1%, respectively, as compared to rats treated with AlCl3 alone (Fig. 4A). In addition, it was shown that AlCl3 injection significantly decreased the pGSK-3β (Ser 9) level by 81.9% compared to the control group. Nevertheless, AlCl3 + BAs (125 mg/kg) or AlCl3 + BAs (250 mg/kg) treatments significantly increased pGSK-3β (Ser 9) levels as compared to rats treated with AlCl3 only (Fig. 4B). Moreover, the effect of different treatment groups on β-catenin levels is displayed in Fig. 4C. AlCl3 exhibited a significant decrease in β-catenin level by 86% compared to the control group. However, in the two used doses, BAs significantly increased β-catenin levels compared to the AlCl3-treated group.
Effect of Boswellic acids on Alzheimer’s-induced alterations in the hippocampal brain-derived neurotrophic factor, p-glycogen synthase kinase-3 beta, and beta-catenin levels. A brain-derived neurotrophic factor, B p-glycogen synthase kinase-3 beta, C beta-catenin. Data are mean ± SD (n = 8). a, b, or c: significantly different compared to the control, AD group, or BAs (125 mg/kg) group respectively, P < 0.05 using ANOVA followed by Tukey’s as post hoc test. AD Alzheimer’s group (AlCl3 (100 mg/kg)), BAs Boswellic acids
BAs (250 mg/kg) exhibited a more potent effect in all the measured biochemical parameters than BAs (125 mg/kg).
Discussion
Recently, natural compounds have received much attention as an alternative or adjuvant therapy in treating NDs. Many of them have been traditionally used to improve learning and memory. Frankincense is the only recognized source of BAs responsible for the pharmacological properties of the gum resin of Boswellia serrata. This resin was described in ancient Ayurvedic scripts as a therapy for many inflammatory disorders. Lately, many studies have reported different pharmacological effects of the crude extract and BAs owing to their anti-inflammatory, antioxidant, anti-nociceptive, anti-bacterial, anti-arthritis, and neuroprotective properties (Al-Harrasi et al. 2019). Regarding its neuroprotective effects, traditionally, Boswellia serrata is recommended by Avicenna for pregnant women to improve the memory of their infants and for aged people to inhibit amnesia (Wynn and Fougère 2007).
The relationship between Al and AD was documented. Al deposits in different brain regions impairs memory and cognitive functions and boosts the deposition of Aβ, lipid peroxidation, and aggregation of hyperphosphorylated tau (Saba et al. 2017). In our study, the neurotoxic effects of AlCl3 were illustrated, where AlCl3-intoxicated rats showed increased AChE level with subsequent impairment of memory and cognition, augmented Aβ formation, induced oxidative stress, enhanced the expression of pro-inflammatory mediators and neuronal damage, as reported by Saba et al. (2017) and Justin Thenmozhi et al. (2017). Moreover, impairment of cognition, memory, and spatial learning was also recorded in the AlCl3-treated group. On the contrary, Bas-treated rats showed improvements in these behavioral abnormalities. Based on the literature, either B. serrata as an extract or isolated BAs can enhance memory and cognitive functions (Mahmoudi et al. 2011; Karima et al. 2012; Majdinasab et al. 2016; Ebrahimpour et al. 2017), which is explained based on the anti-inflammatory and antioxidant effects of BAs. In addition, decreased ACh level due to increased AChE activity participates in cognitive worsening. Accordingly, reduced AChE activity in BAs-treated groups was accompanied by improvements in abnormal behavior in OFT and MWM tests in harmony with Yassin et al. (2013) and Ebrahimpour et al. (2017).
Oxidative stress is the main contributor to the harmful effects of NDs. Therefore, the antioxidant treatment that reduces ROS is a promising key to decreasing AD progression. Lipid peroxide formation was elevated, while SOD and TAC levels were reduced in AlCl3-treated rats, as reported by Mohamed et al. (2021). Conversely, co-treatment with BAs restored the oxidative status. The antioxidant properties of BAs have been reported in different studies (Umar et al. 2014; Rajabian et al. 2020). A previous study by Ebrahimpour et al. (2017) said that BAs improve cognitive function and exhibit neuroprotective effects through their antioxidant action (Ebrahimpour et al. 2017).
Increased ROS production contributes to amyloid aggregation inside different brain regions. Aβ formation is the starting point for a long chain of pathophysiological actions in AD. Aβ aggregation activates excessive production of proinflammatory cytokines such as TNF-α and IL-1β, which also enhances a lot of Aβ overproduction as illustrated in our rat model. On the other hand, BAs significantly hinder Aβ aggregation and decrease TNF-α and IL-1β. The anti-amyloid effects of BAs can be explained in terms of their antioxidant and anti-inflammatory activities as neuroinflammation is considered one of the essential keys involved in NDs progression; drugs that have anti-inflammatory activities are supposed to treat or at least delay the progression. Our results agree with studies reporting the anti-inflammatory effect of BAs in different models (Shehata et al. 2011; Ammon 2019). Umar et al. (2014) said that B. serrata extract has an immune-modulatory effect and can be used to remedy chronic inflammatory diseases such as arthritis (Umar et al. 2014). Moreover, BAs reduce Aβ deposition and the cognitive dysfunction induced by lipopolysaccharide injection through its anti-inflammatory effect (Sayed and El Sayed 2016; Sayed et al. 2018).
The harmful effects of Aβ overexpression extend to reducing the expression of BDNF. BDNF is a master neurotrophin implicated in learning and memory, neurogenesis, synaptic plasticity, and dendritic density of the neurons. BDNF level is decreased in AD patients, accompanied by learning and memory impairment (Xie et al. 2017). So, to enhance memory and learning abilities, the BDNF level should be restored. The inhibition of proinflammatory mediators is also implicated in the neuroprotective property of BDNF (Fang et al. 2019). In our study, BAs reinstated BDNF level with subsequent enhancement of memory functions. The anti-amyloidogenic action of BAs may explain this result and its ability to hinder inflammation and oxidative stress. Despite traditional and pharmacological reports indicating the neuroprotective effects of Boswellia gum resin and its ability to enhance memory power, gaps are still present, specifically in the molecular mechanisms which underline its protective effects. It was reported by Hosseini et al. (2010) that treatment of rats with an aqueous extract of Boswellia enhances the spatial memory, and this effect is partly due to upregulation of BDNF and not via BDNF-CREB-BDNF cycle and suggests there is another pathway related to BDNF (Hosseini et al. 2010). It has been recently reported that BDNF applies its neurotrophic effects via crosstalk with the Wnt/β-catenin signaling pathway, and GSK-3β mediated this interaction and acted as the primary mediating crosstalk factor (Yang et al. 2016; Xie et al. 2017; Fang et al. 2019; Zhang et al. 2019). GSK-3β, a serine/threonine-protein kinase, controls many physiological functions. Its activity is regulated by phosphorylation with other proteins. Active GSK-3β is phosphorylated at (Tyr216), while phosphorylation at (Ser9) turns it inactive. Therefore, agents that can upregulate p-GSK-3β (Ser9) may be suggested to treat AD (Jaworski et al. 2019). GSK-3β, as a kinase, constantly phosphorylates different signaling molecules and transcription factors, one of these critical transcription factors is β-catenin. Phosphorylation of β-catenin by GSK-3β causes proteasomal degradation and discourages the translocation of β-catenin to the nucleus. Conversely, inhibition of GSK-3β catalytic activity allowed β-catenin to accumulate and migrate to the heart, where β-catenin encouraged de novo synthesis of essential growth factors and neurotrophins as BDNF (Yang et al. 2016; Libro et al. 2016). In addition, upregulation of BDNF enhances this pathway and inhibits GSK-3β activity (Yang et al. 2016). Furthermore, activation of Wnt signaling prevents Aβ production; in contrast, dysfunction of Wnt signaling promotes Aβ production and aggregation (Jia et al. 2019a). From the identified BDNF/GSK-3β/β-catenin axis, dysfunction of Wnt signaling is enough to encourage a neuropathological process incorporated in AD as cognitive impairment and aggregation of Aβ. Wnt/β-catenin signaling cascade is also a pathway for neuroprotection through suppression of oxidative stress, Aβ production, inflammation, and apoptosis (Xian et al. 2016; Vallée et al. 2017). Additionally, Wang et al. (2019) revealed that glutamine reduced oxidative stress-induced AD via activating the Wnt3/β-catenin signaling pathway. In the current study, the effect of BAs on the players in this cascade GSK-3β, BDNF, and β-catenin was investigated (Wang et al. 2019). In our rat AD model, we found that the p-GSK-3β (Ser9) level was decreased (i.e., increased GSK-3β activity) with a subsequent decrease in the total hippocampal β-catenin and BDNF levels. However, treatment with BAs upregulated GSK-3β (Ser9), which suppressed the activity of GSK-3β, increased β-catenin level, and enhanced BDNF production in the hippocampus. Regulation of the Wnt/β-catenin signaling cascade is not merely reflected in the memory and cognition (Hui et al. 2018) but also reflected in Aβ production and aggregation (Jia et al. 2019a), the inflammation, and oxidative status (Xian et al. 2016; Vallée et al. 2017) as explored in the current study. Up to date, few studies reported the effect of BAs on the Wnt/β-catenin signaling cascade, one regarding bone regeneration (Xiong et al. 2019) and others related to its anti-tumor activity (Liu et al. 2013). Recently, Gomaa et al. (2019) illustrated the effect of B. serrata on GSK-3β activity and its contribution to improving cognitive dysfunction in diabetic rats (Gomaa et al. 2019).
In conclusion, BAs have neuroprotective effects against AlCl3-induced AD symptoms, and this effect may be mediated, to some extent, through the anti-amyloidogenic, anti-inflammatory, and antioxidant activities of BAs. The probable mechanisms underlying the potential neuroprotective activity of BAs may also be attributed to their modulatory effect of the Wnt/β-catenin signaling cascade.
Data avaiability
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- Aβ:
-
Amyloid-β
- AD:
-
Alzheimer’s disease
- BA:
-
Bosweillic acids
- BDNF:
-
Brain-derived neurotrophic factor
- NDs:
-
Neurodegenerative diseases
- GSK-3β:
-
Glycogen synthase kinase 3β
References
Al-Harrasi A, Hussain H, Csuk R, Khan HY (2019) Chapter 4—Biological activities of Boswellia extract. In: Al-Harrasi A, Hussain H, Csuk R, Yar Khan H (eds) Chemistry and bioactivity of Boswellic acids and other terpenoids of the genus Boswellia. Elsevier, pp 111–125
Ameen AM, Elkazaz AY, Mohammad HMF, Barakat BM (2017) Anti-inflammatory and neuroprotective activity of boswellic acids in rotenone parkinsonian rats. Can J Physiol Pharmacol 95:819–829. https://doi.org/10.1139/cjpp-2016-0158
Ammon HPT (2019) Boswellic extracts and 11-keto-ß-boswellic acids prevent type 1 and type 2 diabetes mellitus by suppressing the expression of proinflammatory cytokines. Phytomedicine 63:153002. https://doi.org/10.1016/j.phymed.2019.153002
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Byler KG, Setzer WN (2018) Protein targets of frankincense: a reverse docking analysis of terpenoids from Boswellia oleo-gum resins. Medicines (Basel) 5. https://doi.org/10.3390/medicines5030096
Colomina MT, Peris-Sampedro F (2017) Aluminum and Alzheimer’s Disease Adv Neurobiol 18:183–197. https://doi.org/10.1007/978-3-319-60189-2_9
Cunha JM, Masur J (1978) Evaluation of psychotropic drugs with a modified open field test. Pharmacology 16:259–267. https://doi.org/10.1159/000136777
Ebrahimpour S, Fazeli M, Mehri S et al (2017) Boswellic acid improves cognitive function in a rat model through its antioxidant activity: - neuroprotective effect of Boswellic acid. J Pharmacopuncture 20:10–17. https://doi.org/10.3831/KPI.2017.20.001
Efferth T, Oesch F (2020) Anti-inflammatory and anti-cancer activities of frankincense: targets, treatments and toxicities. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.01.015
Fang W, Liao W, Zheng Y et al (2019) Neurotropin reduces memory impairment and neuroinflammation via BDNF/NF-κB in a transgenic mouse model of Alzheimer’s disease. Am J Transl Res 11:1541–1554
Fu P, Yung KKL (2020) Air pollution and Alzheimer’s disease: a systematic review and meta-analysis. Journal of Alzheimer’s Disease 77:701–714. https://doi.org/10.3233/JAD-200483
Gomaa AA, Makboul RM, Al-Mokhtar MA, Nicola MA (2019) Polyphenol-rich Boswellia serrata gum prevents cognitive impairment and insulin resistance of diabetic rats through inhibition of GSK3β activity, oxidative stress, and pro-inflammatory cytokines. Biomed Pharmacother 109:281–292. https://doi.org/10.1016/j.biopha.2018.10.056
Hosseini M, Hadjzadeh MA-R, Derakhshan M et al (2010) The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in Morris water maze. Arch Pharm Res 33:463–468. https://doi.org/10.1007/s12272-010-0317-z
Hui J, Zhang J, Pu M et al (2018) Modulation of GSK-3β/β-catenin signaling contributes to learning and memory impairment in a rat model of depression. Int J Neuropsychopharmacol 21:858–870. https://doi.org/10.1093/ijnp/pyy040
Jalili C, Salahshoor MR, Moradi S et al (2014) The therapeutic effect of the aqueous extract of boswellia serrata on the learning deficit in kindled rats. Int J Prev Med 5:563–568
Jaworski T, Banach-Kasper E, Gralec K (2019) GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural Plast 2019:4209475. https://doi.org/10.1155/2019/4209475
Jia L, Piña-Crespo J, Li Y (2019a) Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol Brain 12:104. https://doi.org/10.1186/s13041-019-0525-5
Jia R-X, Liang J-H, Xu Y, Wang Y-Q (2019b) Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr 19:181. https://doi.org/10.1186/s12877-019-1175-2
Justin Thenmozhi A, William Raja TR, Manivasagam T et al (2017) Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr Neurosci 20:360–368. https://doi.org/10.1080/1028415X.2016.1144846
Karima O, Riazi G, Khodadadi S et al (2012) An in vitro study of the role of β-boswellic acid in the microtubule assembly dynamics. FEBS Lett 586:4132–4138. https://doi.org/10.1016/j.febslet.2012.10.007
Libro R, Bramanti P, Mazzon E (2016) The role of the Wnt canonical signaling in neurodegenerative diseases. Life Sci 158:78–88. https://doi.org/10.1016/j.lfs.2016.06.024
Liu H-P, Gao Z-H, Cui S-X et al (2013) Chemoprevention of intestinal adenomatous polyposis by acetyl-11-keto-beta-boswellic acid in APC(Min/+) mice. Int J Cancer 132:2667–2681. https://doi.org/10.1002/ijc.27929
Magalingam KB, Radhakrishnan A, Ping NS, Haleagrahara N (2018) Current concepts of neurodegenerative mechanisms in Alzheimer’s disease. Biomed Res Int 2018:3740461. https://doi.org/10.1155/2018/3740461
Mahmoudi A, Hosseini-Sharifabad A, Monsef-Esfahani HR et al (2011) Evaluation of systemic administration of Boswellia papyrifera extracts on spatial memory retention in male rats. J Nat Med 65:519–525. https://doi.org/10.1007/s11418-011-0533-y
Majdinasab N, Siahpush A, Mousavinejad SK et al (2016) Effect of Boswellia serrata on cognitive impairment in multiple sclerosis patients. Journal of Herbal Medicine 6:119–127. https://doi.org/10.1016/j.hermed.2016.05.003
Mohamed EA, Ahmed HI, Zaky HS, Badr AM (2021) Sesame oil mitigates memory impairment, oxidative stress, and neurodegeneration in a rat model of Alzheimer’s disease. A pivotal role of NF-κB/p38MAPK/BDNF/PPAR-γ pathways. J Ethnopharmacol 267:113468. https://doi.org/10.1016/j.jep.2020.113468
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60. https://doi.org/10.1016/0165-0270(84)90007-4
Peden AH, Ironside JW (2012) Molecular pathology in neurodegenerative diseases. Curr Drug Targets 13:1548–1559. https://doi.org/10.2174/138945012803530134
Rajabian A, Sadeghnia H, Fanoudi S, Hosseini A (2020) Genus Boswellia as a new candidate for neurodegenerative disorders. Iran J Basic Med Sci 23:277–286. https://doi.org/10.22038/IJBMS.2020.35288.8419
Saba K, Rajnala N, Veeraiah P et al (2017) Energetics of excitatory and inhibitory neurotransmission in aluminum chloride model of Alzheimer’s disease: reversal of behavioral and metabolic deficits by Rasa Sindoor. Front Mol Neurosci 10:323. https://doi.org/10.3389/fnmol.2017.00323
Sayed AS, El Sayed NSED (2016) Co-administration of 3-acetyl-11-keto-beta-boswellic acid potentiates the protective effect of celecoxib in lipopolysaccharide-induced cognitive impairment in mice: possible implication of anti-inflammatory and antiglutamatergic pathways. J Mol Neurosci 59:58–67. https://doi.org/10.1007/s12031-016-0734-7
Sayed AS, Gomaa IEO, Bader M, El Sayed NSED (2018) Role of 3-acetyl-11-keto-beta-boswellic acid in counteracting LPS-induced neuroinflammation via modulation of miRNA-155. Mol Neurobiol 55:5798–5808. https://doi.org/10.1007/s12035-017-0801-2
Shehata AM, Quintanilla-Fend L, Bettio S et al (2011) Prevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE). Phytomedicine 18:1037–1044. https://doi.org/10.1016/j.phymed.2011.06.035
Umar S, Umar K, Sarwar AHMG et al (2014) Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 21:847–856. https://doi.org/10.1016/j.phymed.2014.02.001
Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N (2017) Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim Biophys Sin (shanghai) 49:853–866. https://doi.org/10.1093/abbs/gmx073
Wang Y, Wang Q, Li J et al (2019) Glutamine improves oxidative stress through the Wnt3a/β-catenin signaling pathway in Alzheimer’s disease in vitro and in vivo. Biomed Res Int 2019:4690280. https://doi.org/10.1155/2019/4690280
Wynn SG, Fougère BJ (eds) (2007) Chapter 24—Materia Medica. In: Veterinary herbal medicine. Mosby, Saint Louis, pp 459–672
Xian Y-F, Ip S-P, Mao Q-Q, Lin Z-X (2016) Neuroprotective effects of honokiol against beta-amyloid-induced neurotoxicity via GSK-3β and β-catenin signaling pathway in PC12 cells. Neurochem Int 97:8–14. https://doi.org/10.1016/j.neuint.2016.04.014
Xie B, Xu Y, Liu Z et al (2017) Elevation of peripheral BDNF promoter methylation predicts conversion from amnestic mild cognitive impairment to Alzheimer’s disease: a 5-year longitudinal study. J Alzheimers Dis 56:391–401. https://doi.org/10.3233/JAD-160954
Xiong L, Liu Y, Zhu F et al (2019) Acetyl-11-keto-β-boswellic acid attenuates titanium particle-induced osteogenic inhibition via activation of the GSK-3β/β-catenin signaling pathway. Theranostics 9:7140–7155. https://doi.org/10.7150/thno.35988
Yang J-W, Ma W, Luo T et al (2016) BDNF promotes human neural stem cell growth via GSK-3β-mediated crosstalk with the wnt/β-catenin signaling pathway. Growth Factors 34:19–32. https://doi.org/10.3109/08977194.2016.1157791
Yassin N, El-Shenawy S, Mahdy K et al (2013) Effect of Boswellia serrata on Alzheimer’s disease induced in rats. undefined
Zhang F, Liu C-L, Tong M-M et al (2019) Both Wnt/β-catenin and ERK5 signaling pathways are involved in BDNF-induced differentiation of pluripotent stem cells into neural stem cells. Neurosci Lett 708:134345. https://doi.org/10.1016/j.neulet.2019.134345
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
EM contributed to conceptualization, methodology, validation, writing original draft papers, and writing and review. HA contributed to methodology, validation, writing and review, and supervision. HZ contributed to methodology, validation, data analysis, and review. AB contributed to the methodology and writing of the paper.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted by ethical procedures and policies stated by the Ethics Committee of the Faculty of Pharmacy (Girls), Al-Azhar University that is consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No.8023, revised 1978).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Lotfi Aleya.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 6355 KB)
Supplementary file3 (MP4 2594 KB)
Supplementary file4 (MP4 11537 KB)
Supplementary file5 (MP4 16954 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, E.A., Ahmed, H.I., Zaky, H.S. et al. Boswellic acids ameliorate neurodegeneration induced by AlCl3: the implication of Wnt/β-catenin pathway. Environ Sci Pollut Res 29, 76135–76143 (2022). https://doi.org/10.1007/s11356-022-20611-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20611-5