Abstract
Sentinel lymph node (SLN) sampling is important for evaluating the nodal stage of breast cancer when the axillary nodes are clinically free of metastasis. The intraoperative frozen section (IFS) of SLN is used for lymph node assessment. This meta-analysis aims to provide evidence about the diagnostic accuracy and the applicability of IFS of SLN in breast cancer patients. Data were collected by searching PubMed, Cochrane, Scopus, and Web of Science electronic databases for trials matching our eligibility criteria. The statistical analysis included the sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and pooled studies’ diagnostic odds ratio outcomes. The analyses were conducted using the Open Meta-analyst software. This meta-analysis pooled the results of 110 studies. The overall sensitivity of IFS for SLN metastasis was 74.7%; 95% CI [72.0, 77.2], P < 0.001. It was 31.4% 95% CI [25.2, 38.3], P < 0.001 for the micro-metastasis, and 90.2%; 95% CI [86.5, 93.0], P < 0.001 for the macro-metastasis. The overall specificity was 99.4%; 95% CI [99.2, 99.6], P < 0.001. The overall positive likelihood ratio was 121.4; 95% CI [87.9, 167.6], P < 0.001, and the overall negative likelihood ratio was 0.226; 95% CI [0.186, 0.274], P < 0.001. The overall diagnostic odds ratio of IFS for diagnosing SLN metastasis was 569.5; 95% CI [404.2, 802.4], P < 0.001. The intraoperative frozen section of SLN has good sensitivity for diagnosing breast cancer macro-metastasis. However, the sensitivity is low for micro-metastasis. The specificity is very satisfactory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently cancer statistics stated that breast cancer has the highest incidence among all cancers in women. In 2020, more than 680,000 died from breast cancer worldwide (Sung et al. 2021). Due to recently developed screening programs and elevated public awareness of breast cancer, the early detection rate has increased; therefore, axillary metastases’ incidence rates are continuously decreasing (Berry et al. 2005).
The sentinel lymph node biopsy (SLNB) technique is routinely used for nodal staging of breast cancer when nodal metastasis is not manifested clinically. Lymph node evaluation is essential because axillary lymph nodes’ status affects survival and the cancer recurrence rate more than any other factor (Fisher et al. 1983). SLNB has a lower incidence of complications, especially lymphedema, than axillary lymph node dissection (ALND). This limits the use of ALND only to patients with metastatic sentinel lymph nodes (SLN) (Cipolla et al., 2010; Lucci et al. 2007; Peintinger et al. 2003; Veronesi et al. 2003).
Intraoperative frozen section (IFS) is one of the most commonly used methods for intraoperative assessment of SLN. If intraoperative SLN is positive, patients will proceed directly for immediate ALND, thus sparing them from the burden of a second operation which may be more complex, time-consuming, and carry greater risks from anesthesia or other possible complications (Veronesi et al. 2003).
The American College of Surgeons Oncology Group Z0011 trial (ACOSOGZ0011) started in the late 1990s and the International Breast Cancer Study Group 23–01 trial (IBCSG 23–01) started in 2001 revised the indications to perform ALND in positive SLN patients. As many positive SLN cancer patients do not have additional metastatic lymph nodes at the ALND, the ACOSOG Z0011 trial randomized patients with T1 to T2 tumors and (1–2) positive SLNs who underwent conservative breast surgery with whole-breast irradiation to either complete the ALND or to not undergo any further axillary surgery. ACOSOGZ0011 showed no differences in overall 10-year survival between the patients treated with ALND and those treated with SLNB alone with less morbidity in SLNB. Also, results from IBCSG 23–01 after a 10-year follow-up showed that ALND could be safely carried out in T1 to T2 breast cancer patients with SLN micro-metastases (Baron et al. 2007; Galimberti et al. 2013; Giuliano 2011; Giuliano et al. 2017). According to the American Society of Clinical Oncology, IFS is the recommended method for the intraoperative evaluation of SLNs. However, IFS may cause some destruction to the diagnostic tissue (Lyman et al. 2005).
Several studies investigated the applicability of IFS in detecting macro-metastasis (MAM) and micro-metastasis (Mi) of SLNs, but with significant variability in their samples and results. Furthermore, many studies have been published since the last meta-analysis determined the IFS applicability, so we aim to provide a current, complete vision about the overall accuracy and applicability of IFS of SLNs in breast cancer patients.
Methods
We adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Liberati et al., 2009). We followed the guidelines of the “Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy” (Macaskill et al., 2010).
Literature search
We searched the published literature on PubMed, Cochrane, Scopus, and Web of Science databases using the following keywords: “sentinel lymph node,” “SLN,” “frozen section biopsy,” “breast cancer,” “breast neoplasm,” “mammary cancer,” “breast tumor,” and “breast carcinoma.” The last search update was in January 2021. After removing duplicates by Endnote, four authors screened titles and abstracts of retrieved records according to our eligibility criteria. Then, potentially eligible articles underwent full-text screening to confirm their eligibility for the meta-analysis. In addition, we searched references of included studies manually for additional relevant articles according to our eligibility criteria. Any discrepancy among authors was solved by discussion and consensus. Two reviewers revised the screening process to ensure that all eligible studies were included.
Study eligibility criteria
We included observational and interventional trials on breast cancer that enrolled patients with no clinical manifestations of nodal metastasis. Studies that compared the diagnostic accuracy of IFS for SLN metastasis with that of definitive histopathology were included. We applied no restrictions concerning language, publication date, place, or age. For overlapping datasets, we included the articles reporting the most complete data set. We excluded articles with missing sensitivity or specificity data. We also excluded reviews, letters, editorials, conference papers, and animal studies.
Data extraction
Five authors extracted the following data items in a separate Excel sheet: (1) summary of the included studies, including design, sample size, number of SLNB, reference comparator, and conclusions; (2) baseline criteria of included population, including age, mean number of SLN, radiology tumor size, histologic type, estrogen and progesterone receptors, human epidermal growth factor receptor 2, lympho-vascular invasion, nuclear grade, and type of metastasis; and (3) diagnostic accuracy outcomes, including true positive, true negative, false positive, and false negative. When different authors found differences in extracted data, these disagreements were solved by discussion and consensus.
Quality assessment of included studies
To assess the quality of the included studies, we used the quality assessment tool for the diagnostic accuracy studies (QUADAS) (Whiting et al. 2003). This tool includes the risk of bias and applicability concerns of the following items: (1) patient selection, including three risk of bias domains: random or consecutive sampling, case–control design avoidance, and inappropriate exclusions avoidance; (2) index test, including two risk of bias domains: blinding of the reference standard results during index test result interpretation, and pre-specification of the used threshold if present; and (3) reference standard, including two risk of bias domains: the correct classification of the target condition by the reference standard, and blinding of the index test results during reference standard result interpretation. In addition, the tool includes the risk of bias for another item: (4) flow and timing, including three domains: the appropriate interval between index test and reference standard, including all patients in the analysis, and receiving the same reference standard by all patients. Each risk of bias item was judged as low, high, or unclear risk of bias, and each applicability concern item was judged as low, high, or unclear concern. Five authors assessed the risk of bias independently, and any disagreement was resolved by discussion and a senior reviewer consultation.
Statistical analysis
We used the Open Meta-analyst software to execute our analyses. To assess the diagnostic accuracy of IFS, we calculated its sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with 95% confidence intervals (CIs). Besides, we used the summary receiver operating characteristic (SROC) curve analyses with the sensitivity representing the Y-axis and the 1-specificity representing the X-axis. We pooled the results of included studies using the DerSimonian-Laird method under the random-effects model. We assessed heterogeneity across studies using the chi-square test and evaluated its extent using the I-square test. Heterogeneity was considered significant when the chi-square P-value was less than 0.1 and I2 > 50%. We conducted the analyses for these outcomes on the total sample. In addition, we analyzed the sensitivity and the DOR outcomes for MAM and Mi separately. Isolated tumor cell implants were considered as Mi metastases.
Results
Literature search results
We retrieved 755 unique records from searching databases. After title and abstract screening, 146 studies were subjected to full-text screening. Among these studies, only 110 studies were eligible for the analysis. The PRISMA flow diagram shows the details of the data collection, screening, and study selection process (Fig. 1).
Characteristics of included studies and study population
We included 110 studies (62 prospective and 48 retrospective) with a total of 47,622 patients and about 65,532 SLNB. The mean age of subjects in included studies varied from 45 ± 9 to 65 ± 8 years. Infiltrating ductal carcinoma was the commonest histological type of breast cancer, and most patients had positive estrogen and positive progesterone receptors. Supplementary Table 1 shows the summary of included studies, and Supplementary Table 2 shows the features of their included subjects.
Quality assessment
Regarding the risk of bias, most studies had a low risk of bias concerning index test and flow and timing items. About half of the studies had a low risk of bias as for patient selection. Most studies had an unclear risk of bias about reference standard. Regarding applicability concerns, most studies had a low risk of bias as for index test and an unclear risk of bias as for reference standard. About half of the studies had a low risk of bias regarding patient selection. Figure 2 shows the recap of the quality assessment items, and Supplementary Fig. 1 shows the detailed judgment of each item.
Study outcomes
Sensitivity
The overall sensitivity of IFS for detection of SLN metastasis was 74.7%; 95% CI [72.0, 77.2], P < 0.001 (Fig. 3). Pooled studies were heterogenous (P < 0.001; I2 = 89.5%). The overall sensitivity of IFS for detection of SLN Mi was 31.4%; 95% CI [25.2, 38.3], P < 0.001 (Fig. 4). Pooled studies were heterogenous (P < 0.001; I2 = 81.4%). The overall sensitivity of IFS for detection of SLN MAM was 90.2%; 95% CI [86.5, 93.0], P < 0.001 (Fig. 5). Pooled studies were heterogenous (P < 0.001; I2 = 88.4%).
Specificity
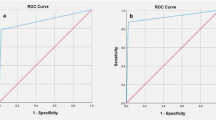
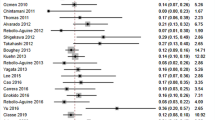
The overall specificity of IFS for detection of SLN metastasis was 99.4%; 95% CI [99.2, 99.6], P < 0.001 (Fig. 6). Pooled studies were heterogenous (p < 0.001; I2 = 48.9%). The SROC curve shows the trade-off between sensitivity and 1-specificcity (Fig. 7).
Positive likelihood ratio
The overall positive likelihood ratio of IFS for detection of SLN metastasis was 121.4; 95% CI [87.9, 167.6], P < 0.001 (Supplementary Fig. 2). Pooled studies were heterogenous (P < 0.001; I2 = 56.5%).
Negative likelihood ratio
The overall negative likelihood ratio of IFS for detection of SLN metastasis was 0.226; 95% CI [0.186, 0.274], P < 0.001 (Supplementary Fig. 2). Pooled studies were homogenous (P = 1; I2 = 0%).
Diagnostic odds ratio
The overall diagnostic odds ratio for diagnosis of SLN metastasis by IFS was 569.5; 95% CI [404.2, 802.4], P < 0.001 (Supplementary Fig. 3). Pooled studies were heterogenous (P < 0.001, I2 = 54%). The overall diagnostic odds ratio for diagnosis of SLN Mi by IFS was 1.347; 95% CI [0.468, 3.882], P = 0.581 (Supplementary Fig. 4). Pooled studies were heterogenous (P < 0.001, I2 = 62.6%). The overall odds ratio for diagnosis of SLN MAM by IFS was 29.245; 95% CI [10.29, 83.114], P < 0.001 (Supplementary Fig. 5). Pooled studies were heterogenous (P < 0.001, I2 = 60.1%).
Discussion
Our analysis showed that the IFS of SLN, which is used to diagnose breast cancer lymph node metastasis, has a sensitivity of 74.7% and a specificity of 99.4%. Patients with positive test results have 121-fold higher odds of having SLN metastasis (positive likelihood ratio), while patients with negative test results have 4.4-fold lower odds of having SLN metastasis (negative likelihood ratio). By subgroup analysis, we also found that sensitivity was 31.4% in terms of micro-metastasis, while regarding macro-metastasis, sensitivity was 90.2%.
When metastasis proceeds, it first affects the axillary lymph nodes (ALNs). Thus, breast cancer may be first suspected by detecting the clinically affected nodes. Therefore, ALN metastasis is considered an indicator of overall recurrence and survival rates (Huston and Simmons 2005). Precise assessment of ALN yields information about the stage of breast cancer or provides instructions concerning treatment options. Surgical management has progressed from radical resection to further advanced procedures and strategies (Coughlin and Ekwueme 2009; Samphao et al. 2008). ALND is a prognostic and therapeutic index that is one of the initial approaches for managing clinically positive nodes in breast cancer patients. However, ALND may result in numerous side effects such as shoulder mobility disorders, wound infections, and seroma formation (Roses et al. 1999). Accordingly, SLNB has displaced ALND in detecting lymph node metastases in order to avoid ALND side effects and complications (Schrenk et al., 2000).
SLNB provides a more accurate diagnostic method with a low false-negative rate and corresponding lower morbidity rates (McMasters et al. 2000). If intraoperative SLN analysis is free from metastasis, ALND can then be avoided, but if the result is positive, ALND is performed during the tumor removal, thus avoiding the need for a second surgery. Moreover, the pathologist can obtain diverse details by examining only a small number of nodes concerning SLN (Cserni et al., 2004, 2003).
Various techniques were mentioned in the literature for intraoperative assessment of SLN, such as IFS analysis, touch imprint cytology (TIC), and rapid cytokeratin immunostaining or combinations of these procedures (Madsen et al. 2012).
An IFS is most often used in oncological surgery such as breast cancer, lung, and endometrial surgeries. Luis Alcazar et al. reported that in patients with endometrial cancer, IFS was superior to intraoperative gross evaluation (IGE) for diagnosing deep myometrial malignant infiltration (Alcazar et al. 2016). Also, IFS may evaluate the extent of local malignant infiltration in patients with lung adenocarcinoma. It has high accuracy and ability to differentiate between pre-/minimally invasive adenocarcinoma (IAC) and IAC (Li et al. 2019).
However, IFS has several disadvantages, including loss of tissue during the sectioning process, tissue architecture alteration, tissue manipulation due to freezing and resoftening of specimens, and the high cost (Martínez García, 2019; Treseler 2006; Varga et al. 2008).
However, using IFS of SLNB in breast cancer is still questionable due to the notable variation in its sensitivity. Previous studies revealed that IFS sensitivity in identifying Mi is low compared to MAM (Morgan et al. 1999; Veronesi et al. 1996; Weiser et al. 2000). In 2012, a previous meta-analysis reported that, by pooling the results of 47 studies comprising 13,062 women with breast cancer, the IFS of SLNs has an outstanding sensitivity for MAM, reaching 94%. However, it was not sensitive enough for Mi at a level of 40%. The mean specificity was 100% (Liu et al. 2011). Our results are similar to their results, with a sensitivity of 31.4% for MAM, 90.2% for Mi, and a specificity level reaching 99.4% that included 47 studies comprising 13,062 women.
Our meta-analysis followed the steps described in the “Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy.” We included many studies with an overall large sample size which increased the generalizability of our results. All study designs were included. We pooled all studies together then subgrouped them into either Mi or MAM to detect the sensitivity for both types. In general, the included studies had a moderate quality. However, our study has some limitations as most of the included studies were observational. Also, significant heterogeneity was detected.
Reliable intraoperative techniques for detecting SLN micro-metastasis are still lacking. We recommend future studies to conduct a network meta-analysis to compare the diagnostic accuracy of different diagnostic techniques.
We concluded that, for the diagnosis of metastasis caused by breast cancer, the sensitivity of IFS has excellent sensitivity (90.2%) for macro-metastasis detection in SLNs, while the sensitivity for the diagnosis of micro-metastasis is lower (31.4%). The overall specificity is satisfying (99.4%).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alcazar JL, Dominguez-Piriz J, Juez L, Caparros M, Jurado M (2016) Intraoperative gross examination and intraoperative frozen section in patients with endometrial cancer for detecting deep myometrial invasion: a systematic review and meta-analysis. Int J Gynecol Cancer 26:407–415
Baron RH, Fey JV, Borgen PI, Stempel MM, Hardick KR, Van Zee KJ (2007) Eighteen sensations after breast cancer surgery: a 5-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Ann Surg Oncol 14:1653–1661
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784–1792
Cipolla C, Cabibi D, Fricano S, Vieni S, Gentile I, Latteri MA (2010) The value of intraoperative frozen section examination of sentinel lymph nodes in surgical management of breast carcinoma. Langenbeck’s Arch Surg 395:685–691
Coughlin SS, Ekwueme DU (2009) Breast cancer as a global health concern. Cancer Epidemiol 33:315–318
Cserni G, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Boecker W, Borisch B, Connolly CE, Decker T, Dervan P, Drijkoningen M, Ellis IO, Elston CW, Eusebi V, Faverly D, Heikkila P, Holland R, Kerner H, Kulka J, Jacquemier J, Lacerda M, Martinez-Penuela J, De Miguel C, Peterse JL, Rank F, Regitnig P, Reiner A, Sapino A, Sigal-Zafrani B, Tanous AM, Thorstenson S, Zozaya E, Fejes G, Wells CA (2004) Discrepancies in current practice of pathological evaluation of sentinel lymph nodes in breast cancer. Result of a questionnaire based survey by the European Working Group for Breast Screening Pathology. J Clin Pathol 57:695–701
Cserni G, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Bussolati G, Boecker W, Borisch B, Connolly CE, Decker T, Dervan P, Drijkoningen M, Ellis IO, Elston CW, Eusebi V, Faverly D, Heikkila P, Holland R, Kerner H, Kulka J, Jacquemier J, Lacerda M, Martinez-Penuela J, De Miguel C, Peterse JL, Rank F, Regitnig P, Reiner A, Sapino A, Sigal-Zafrani B, Tanous AM, Thorstenson S, Zozaya E, Wells CA (2003) Pathological work-up of sentinel lymph nodes in breast cancer. Review of current data to be considered for the formulation of guidelines. Eur J Cancer 39:1654–1667
Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, Foster R, Gardner B, Lerner H, Margolese R, Poisson R, Shibata H, Volk H (1983) Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 52:1551–1557
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay J-R, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol 14:297–305
Giuliano AE (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis. JAMA 305:569–569
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis. JAMA 318:918–918
Huston TL, Simmons RM (2005) Locally recurrent breast cancer after conservation therapy. Am J Surg 189:229–235
Li F, Yang L, Zhao Y, Yuan L, Wang S, Mao Y (2019) Intraoperative frozen section for identifying the invasion status of lung adenocarcinoma: a systematic review and meta-analysis. Int J Surg 72:175–184
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Liu LC, Lang JE, Lu Y, Roe D, Hwang SE, Ewing CA, Esserman LJ, Morita E, Treseler P, Leong SP (2011) Intraoperative frozen section analysis of sentinel lymph nodes in breast cancer patients. Cancer 117:250–258
Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Giuliano AE (2007) Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol 25:3657–3663
Lyman GH, Giuliano AE, Somerfield MR, Benson AB, Bodurka DC, Burstein HJ, Cochran AJ, Cody HS, Edge SB, Galper S, Hayman JA, Kim TY, Perkins CL, Podoloff DA, Sivasubramaniam VH, Turner RR, Wahl R, Weaver DL, Wolff AC, Winer EP (2005) American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 23:7703–7720
Macaskill, P., Gatsonis, C., Deeks, J., Harbord, R. & Takwoingi, Y. 2010. Cochrane handbook for systematic reviews of diagnostic test accuracy. Version 0.9. 0. London: The Cochrane Collaboration, 83.
Madsen EVE, Van Dalen J, Van Gorp J, Van Oort PMP, Van Dalen T (2012) Frozen section analysis of sentinel lymph nodes in patients with breast cancer does not impair the probability to detect lymph node metastases. Virchows Arch 460:69–76
Martínez García, S. 2019. Sentinel Lymph Node Biopsy. Actas Dermo-Sifiliograficas, 110, 425-425.
McMasters KMK, Tuttle TM, Carlson DJ, Brown CM, Noyes RD, Glaser RL, Vennekotter DJ, Turk PS, Tate PS, Sardi A, Cerrito PB, Edwards MJ (2000) Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol 18:2560–2566
Morgan A, Howisey RL, Aldape HC, Patton RG, Rowbotham RK, Schmidt EK, Simrell CR (1999) Initial experience in a community hospital with sentinel lymph node mapping and biopsy for evaluation of axillary lymph node status in palpable invasive breast cancer. J Surg Oncol 72:24–30
Peintinger F, Reitsamer R, Stranzl H, Ralph G (2003) Comparison of quality of life and arm complaints after axillary lymph node dissection vs sentinel lymph node biopsy in breast cancer patients. Br J Cancer 89:648–652
Roses DF, Brooks AD, Harris MN, Shapiro RL, Mitnick J (1999) Complications of level I and II axillary dissection in the treatment of carcinoma of the breast. Ann Surg 230:194–201
Samphao S, Eremin JM, El-Sheemy M, Eremin O (2008) Management of the axilla in women with breast cancer: current clinical practice and a new selective targeted approach. Ann Surg Oncol 15:1282–1296
Schrenk P, Rieger R, Shamiyeh A, Wayand W (2000) Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 88:608–614
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin
Treseler P (2006) Pathologic examination of the sentinel lymph node: what is the best method? Breast J 12:S143–S151
Varga Z, Rageth C, Saurenmann E, Honegger C, Von Orelli S, Fehr M, Fink D, Seifert B, Moch H, Caduff R (2008) Use of intraoperative stereomicroscopy for preventing loss of metastases during frozen sectioning of sentinel lymph nodes in breast cancer. Histopathology 52:597–604
Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, Costa A, De Cicco C, Geraghty JG, Luini A, Sacchini V, Veronesi P (1996) Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 349:1864–1867
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De Cicco C, De Lucia F, Gennari R (2003) A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349:546–553
Weiser MR, Montgomery LL, Susnik B, Tan LK, Borgen PI, Cody HS (2000) Is routine intraoperative frozen-section examination of sentinel lymph nodes in breast cancer worthwhile? Ann Surg Oncol 7:651–655
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3:25
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization: Alaa Ahmed Elshanbary, Mohamed Sayed Zaazouee. Validation: Alaa Ahmed Elshanbary, Anas Zakarya Nourelden. Methodology: Anas Zakarya Nourelden, Khaled Mohamed Ragab. Software: Alaa Abdelsameia Awad, Alaa Abdelsalam. Screening: Youssef Bahaaeldin Darwish, Alaa Saad Isa, Boutheyna Drid, Marwa Gamal Mustafa. Data extraction and curation: Alaa Abdelsameia Awad, Alaa Abdelsalam, Islam H. Ibrahim, Radwa Hamdy Allam, Amira A. Abo Ali. Formal analysis: Alaa Ahmed Elshanbary, Mohamed Sayed Zaazouee. Investigation: Hussah I.M. AlGwaiz, Aeshah A Awaji. Resources: Mousa O. Germoush, Walid Abdel-Aziz. Writing-original draft preparation: Walid Abdel-Aziz, Alaa Abdelsameia Awad, Alaa Abdelsalam, Islam H. Ibrahim, Boutheyna Drid, Ashraf Albrakati, Nehmat Ghaboura, Marina Piscopo. Writing—review and editing: Alaa Ahmed Elshanbary, Youssef Bahaaeldin Darwish, Alaa Saad Isa, Marwa Gamal Mustafa, Radwa Hamdy Allam, Amira A. Abo Ali, Anas Zakarya Nourelden, Khaled Mohamed Ragab, Hussah I.M. AlGwaiz, Aeshah A Awaji, Mousa O. Germoush, Mohamed Sayed Zaazouee. Visualization: Ashraf Albrakati, Nehmat Ghaboura, Marina Piscopo. Supervision: Mohamed Sayed Zaazouee. Project administration: Alaa Ahmed Elshanbary. All authors reviewed the manuscript and approved it for publication.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11356_2022_20569_MOESM1_ESM.pdf
Supplementary Fig. 1: The risk of bias summary, showing each quality assessment item’s judgment in each study. (PDF 135 KB)
11356_2022_20569_MOESM2_ESM.pdf
Supplementary Fig. 2: Forest plots for the pooled negative likelihood ratio (to the left) and positive likelihood ratio (to the right) of intraoperative frozen section biopsy in detecting sentinel lymph node metastasis in breast cancer patients. (PDF 19 KB)
11356_2022_20569_MOESM3_ESM.pdf
Supplementary Fig. 3: A forest blot for the pooled diagnostic odds ratio of intraoperative frozen section biopsy in the detection of sentinel lymph node metastasis in breast cancer patients. (PDF 12 KB)
11356_2022_20569_MOESM4_ESM.pdf
Supplementary Fig. 4: A forest blot for the pooled diagnostic odds ratio of intraoperative frozen section biopsy in the detection of sentinel lymph node micro-metastasis in breast cancer patients. (PDF 7 KB)
11356_2022_20569_MOESM5_ESM.pdf
Supplementary Fig. 5: A forest blot for the pooled diagnostic odds ratio of intraoperative frozen section biopsy in detecting sentinel lymph node macro-metastasis in breast cancer patients. (PDF 7 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elshanbary, A.A., Awad, A.A., Abdelsalam, A. et al. The diagnostic accuracy of intraoperative frozen section biopsy for diagnosis of sentinel lymph node metastasis in breast cancer patients: a meta-analysis. Environ Sci Pollut Res 29, 47931–47941 (2022). https://doi.org/10.1007/s11356-022-20569-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20569-4