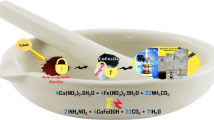
Abstract
The separation of magnetic adsorbents from aqueous solutions is made simple by using an external magnetic field. Herein, magnetic Zr(IV)-ethylenediamine tetramethylene phosphonic acid (EDTMPA) hybrids (MZrOP-x-T, x, and T were the different quality of Fe3O4@C and temperature in the synthesis process, respectively). A study was conducted on the uses of MZrOP-x-T in the capture of U(VI). The influences of pH, adsorption period, initial concentration, and temperature were all investigated. Furthermore, the desorption and reusability of the materials were explored. The optimal values of x and T were 0.2 g and 100 °C, respectively. At 298.15 K, the maximum adsorption capacity of MZrOP-0.2-100 was 330.30 mg·g−1. The current research demonstrates that MZrOP-0.2-100 is a potentially effective material in removing U(VI) from radioactive solution.









Similar content being viewed by others
Change history
11 April 2024
Editor's Note: Readers are alerted that the concerns have been raised with this article. Editorial action will be taken as appropriate once this matter is resolved and all parties have been given an opportunity to respond in full.
References
Barnett MO, Jardine PM, Brooks SC, Selim HM (2000) Adsorption and transport of uranium(VI) in subsurface media. Soil Sci Soc Am J 64:908–917. https://doi.org/10.2136/sssaj2000.643908x
Ben-Tal N, Honig B, Bagdassarian CK, Ben-Shaul A (2000) Association entropy in adsorption processes. Biophys J 79:1180–1187. https://doi.org/10.1016/S0006-3495(00)76372-7
Cai Y, Wu C, Liu Z, Zhang L, Chen L, Wang J, Wang X, Yang S, Wang S (2017) Fabrication of a phosphorylated graphene oxide-chitosan composite for highly effective and selective capture of U(VI). Environ Sci Nano 4:1876–1886. https://doi.org/10.1039/c7en00412e
Cantaluppi C, Degetto S (2000) Civilian and military uses of depleted uranium: environmental and health problems. Annali Di Chimica 90:665–676
Chen L, Wang H, Cao X, Feng Y, Zhang Z, Wang Y, Liu Y (2021) Effects of different phosphorus sources on the adsorption of U(VI) by Zr(IV) organophosphate hybrids. J Solid State Chem 302:122434. https://doi.org/10.1016/j.jssc.2021.122434
Du X, Cheng Y, Liu Z, Yin H, Wu T, Huo L, Shu C (2021) CO2 and CH4 adsorption on different rank coals: a thermodynamics study of surface potential, Gibbs free energy change and entropy loss. Fuel 283:118886. https://doi.org/10.1016/j.fuel.2020.118886
Eichler B, Zvara I (1982) Evaluation of the enthalpy of adsorption from thermochromatographical data. Radiochim Acta 30:233–238. https://doi.org/10.1524/ract.1982.30.4.233
Feng Y, Liu Y, Xue L, Sun H, Guo Z, Zhang Y, Yang L (2017) Carboxylic acid functionalized sesame straw: a sustainable cost-effective bioadsorbent with superior dye adsorption capacity. Bioresour Technol 238:675–683. https://doi.org/10.1016/j.biortech.2017.04.066
Gok C, Aytas S (2009) Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375. https://doi.org/10.1016/j.jhazmat.2009.02.063
Grosvenor AP, Kobe BA, Biesinger MC, McIntyre NS (2004) Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf Interface Anal 36:1564–1574. https://doi.org/10.1002/sia.1984
Han LJ, Ge FY, Sun GH, Gao XJ, Zheng HG (2019a) Effective adsorption of Congo red by a MOF-based magnetic material. Dalton Trans 48:4650–4656. https://doi.org/10.1039/C9DT00813F
Han X, Wang Y, Cao X, Dai Y, Liu Y, Dong Z, Zhang Z, Liu Y (2019b) Adsorptive performance of ship-type nano-cage polyoxometalates for U(VI) in aqueous solution. Appl Surf Sci 484:1035–1040. https://doi.org/10.1016/j.apsusc.2019.04.121
Hebenstreit ELD, Hebenstreit W, Diebold U (2000) Adsorption of sulfur on TiO2(110) studied with STM, LEED and XPS: temperature-dependent change of adsorption site combined with O–S exchange. Surf Sci 461:87–97. https://doi.org/10.1016/S0039-6028(00)00538-0
Hong X, Zhu S, Xia M, Du P, Wang F (2022) Investigation of the efficient adsorption performance and adsorption mechanism of 3D composite structure La nanosphere-coated Mn/Fe layered double hydrotalcite on phosphate. J Colloid Interf Sci 614:478–488. https://doi.org/10.1016/j.jcis.2022.01.149
Hu R, Xiao J, Wang T, Chen G, Chen L, Tian X (2020) Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem Eng J 379:122388. https://doi.org/10.1016/j.cej.2019.122388
Huang L, Li Z, Li R, Wu H (2016) Comparative study of phosphorus adsorption behaviors in lake sediments over short and long periods of time: implication for the prediction of the release of phosphorus by CaCl2 and NaHCO3 extraction. Environ Sci Pollut R 23(24):25145–25155. https://doi.org/10.1007/s11356-016-7755-1
Jasni MJF, Arulkumar M, Sathishkumar P, Yusoff ARM, Buang NA, Gu FL (2017) Electrospun nylon 6, 6 membrane as a reusable nano-adsorbent for bisphenol A removal: adsorption performance and mechanism. J Colloid Interf Sci 508:591–602. https://doi.org/10.1016/j.jcis.2017.08.075
Jia Y, Zhang Y, Wang R, Yi J, Feng X, Xu Q (2012) Mesoporous zirconium phosphonate hybrid material as adsorbent to heavy metal ions. Ind. Eng Chem Res 51(38):12266–12273. https://doi.org/10.1021/ie300253z
Jiao Z, Meng Y, He C, Yin X, Wang X, Wei Y (2021) One-pot synthesis of silicon-based zirconium phosphate for the enhanced adsorption of Sr (II) from the contaminated wastewater. Microporous Mesoporous Mater 318:111016. https://doi.org/10.1016/j.micromeso.2021.111016
Khan SA, Siddiqui WA, Khan AA (2007) Synthesis, characterization and ion-exchange properties of a new and novel ‘organic–inorganic’ hybrid cation-exchanger: nylon-6, 6, Zr (IV) phosphate. Talanta 71(2):841–847. https://doi.org/10.1016/j.talanta.2006.05.042
Komkov NI, Selin VS, Tsukerman VA, Goryachevskaya ES (2017) Problems and perspectives of innovative development of the industrial system in Russian Arctic regions. Stud Russ Econ Dev 28:31–38. https://doi.org/10.1134/S1075700717010051
Ladeira ACQ, Morais CAD (2005) Uranium recovery from industrial effluent by ion exchange—column experiments. Miner Eng 18:1337–1340. https://doi.org/10.1016/j.mineng.2005.06.012
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Liao Q, Li L, Yuan Y, Cheng B, Lu W, Hou S (2017) Preparation of 4-sulfonylcalix[6]arene modified Fe3O4 as adsorbent for adsorption of U(VI) from aqueous solution. J Radioanal Nucl Chem 315:251–261. https://doi.org/10.1007/s10967-017-5650-y
Lin XZ, Ma TY, Yuan ZY (2011) Titania–silica–phosphonate triconstituent hybrid mesoporous materials as adsorbents in gas and liquid phases. Chem Eng J 166(3):1144–1151. https://doi.org/10.1016/j.cej.2010.11.086
Luca V, Hanna JV (2015) A versatile Zr(IV)-organophosphonate coordination polymer platform for the selective adsorption of lanthanides and actinides. Hydrometallurgy 154:118–128. https://doi.org/10.1016/j.hydromet.2015.04.002
Ma TY, Zhang XJ, Shao GS, Cao JL, Yuan ZY (2008) Ordered macroporous titanium phosphonate materials: synthesis, photocatalytic activity, and heavy metal ion adsorption. J Phys Chem C 112(8):3090–3096. https://doi.org/10.1021/jp710636x
Ma TY, Lin XZ, Yuan ZY (2010) Periodic mesoporous titanium phosphonate hybrid materials. J Mater Chem 20(35):7406–7415. https://doi.org/10.1039/c0jm01442g
Maity D, Agrawal DC (2007) Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J Magn Magn Mater 308:46–55. https://doi.org/10.1016/j.jmmm.2006.05.001
Minitha CR, Lalitha M, Jeyachandran YL, Senthilkumar L, Kumar RTR (2017) Adsorption behaviour of reduced graphene oxide towards cationic and anionic dyes: co-action of electrostatic and π–π interactions. Mater Chem Phys 194:243–252. https://doi.org/10.1016/j.matchemphys.2017.03.048
Mu W, Chen B, Yu Q, Li X, Wei H, Yang Y, Peng S (2021) A novel zirconium phosphonate adsorbent for highly efficient radioactive cesium removal. J Mol Liq 326:115307. https://doi.org/10.1016/j.molliq.2021.115307
Nie X, Dong F, Liu M, Sun S, Yang G, Zhang W, Qin Y, Ma J, Huang R, Gong J (2016) Removel of uranium from aqueous solutions by spirodela punctata as the mechanism of biomineralization. Procedia Environ 31:382–391. https://doi.org/10.1016/j.proenv.2016.02.060
Oliveira LCA, Rios RVRA, Fabris JD, Garg V, Sapag K, Lago RM (2002) Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon 40:2177–2183. https://doi.org/10.1016/S0008-6223(02)00076-3
Pérez-Calderón J, Scian A, Ducos M, Santos V, Zaritzky N (2021) Performance of oxalic acid-chitosan/alumina ceramic biocomposite for the adsorption of a reactive anionic azo dye. Environ Sci Pollut R 28(47):67032–67052. https://doi.org/10.1007/s11356-021-15123-7
Pichot R, Spyropoulos F, Norton IT (2012) Competitive adsorption of surfactants and hydrophilic silica particles at the oil-water interface: interfacial tension and contact angle studies. J Colloid Interface Sci 377:396–405. https://doi.org/10.1016/j.jcis.2012.01.065
Pourbeyram S (2016) Effective removal of heavy metals from aqueous solutions by graphene oxide–zirconium phosphate (GO–Zr-P) nanocomposite. Ind Eng Chem Res 55:5608–5617. https://doi.org/10.1021/acs.iecr.6b00728
Quinn JE, Wilkins D, Soldenhoff KH (2013) Solvent extraction of uranium from saline leach liquors using DEHPA/Alamine 336 mixed reagent. Hydrometallurgy 134-135:74–79. https://doi.org/10.1016/j.hydromet.2013.01.014
Rahman N, Haseen U (2014) Equilibrium modeling, kinetic, and thermodynamic studies on adsorption of Pb (II) by a hybrid inorganic–organic material: polyacrylamide zirconium (IV) iodate. Ind Eng Chem Res 53(19):8198–8207. https://doi.org/10.1021/ie500139k
Ren W, Ai Z, Jia F, Zhang L, Fan X, Zou Z (2007) Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl Catal 69:138–144. https://doi.org/10.1016/j.apcatb.2006.06.015
Sheng T, Zhang Z, Hu Y, Tao Y, Zhang J, Shen Z, Feng J, Zhang A (2019) Adsorption of phosphorus by using magnetic Mg–Al-, Zn–Al-and Mg–Fe-layered double hydroxides: comparison studies and adsorption mechanism. Environ Sci Pollut R 26(7):7102–7114. https://doi.org/10.1007/s11356-019-04191-5
Singh S, Wu C, Williams PT (2012) Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J Anal Appl Pyrol 94:99–107. https://doi.org/10.1016/j.jaap.2011.11.011
Skvortsov AM, Gorvunov AA (1986) Adsorption effects in the chromatography of polymers. J Chromatogr 358:77–83. https://doi.org/10.1016/S0021-9673(01)90317-6
Sun H, Yang B, Zhu Z, Yin W, Sheng Q, Hou Y, Yao J (2021) New insights into selective-depression mechanism of novel depressant EDTMPAS on magnesite and quartz surfaces: adsorption mechanism, DFT calculations, and adsorption model. Miner Eng 160:106660. https://doi.org/10.1016/j.mineng.2020.106660
Totemeier TC (2000) Characterization of uranium corrosion products involved in a uranium hydride pyrophoric event. J Nucl Mater 278:301–311. https://doi.org/10.1016/S0022-3115(99)00245-7
Vasylyev MV, Wachtel EJ, Popovitz-Biro R, Neumann R (2006) Titanium phosphonate porous materials constructed from dendritic tetraphosphonates. Chem Eur J 12(13):3507–3514. https://doi.org/10.1002/chem.200501143
Veliscek-Carolan J, Rawal A, Luca V, Hanley TL (2017) Zirconium phosphonate sorbents with tunable structure and function. Microporous Mesoporous Mater 252:90–104. https://doi.org/10.1016/j.micromeso.2017.05.059
Wade CR, Corrales-Sanchez T, Narayan TC, Dincă M (2013) Postsynthetic tuning of hydrophilicity in pyrazolate MOFs to modulate water adsorption properties. Energy Environ 6:2172–2177. https://doi.org/10.1039/c3ee40876k
Wang X, Peng G, Yang Y, Wang Y, He T (2012a) Uranium adsorption by dry and wet immobilized Saccharomyces cerevisiae. J Radioanal Nucl Chem 291:825–830. https://doi.org/10.1007/s10967-011-1358-6
Wang YQ, Zhang ZB, Liu YH, Cao XH, Liu YT, Li Q (2012b) Adsorption of U(VI) from aqueous solution by the carboxyl-mesoporous carbon. Chem Eng J 198-199:246–253. https://doi.org/10.1016/j.cej.2012.05.112
Wang L, Li Z, Wu Q, Huang Z, Yuan L, Chai Z, Shi W (2020a) Layered structure-based materials: challenges and opportunities for radionuclide sequestration. Environ Sci Nano 7:724–752. https://doi.org/10.1039/c9en01429b
Wang Y, Cui W, Wang H, Xie Y, Zhang Z, Cao X, Liu Y (2020b) Adsorption of U (VI) on sulfonated ordered mesoporous polymer. J. Radioanal. Nucl Chem 326:349–360. https://doi.org/10.1007/s10967-020-07322-2
Wang Z, Hu H, Huang L, Lin F, Liu S, Wu T, Alharbi NS, Rabah SO, Lu Y, Wang X (2020c) Graphene aerogel capsulated precipitants for high efficiency and rapid elimination of uranium from water. Chem Eng J 396:125272. https://doi.org/10.1016/j.cej.2020.125272
Wang ML, Zhao Z, Lin S, Su M, Liang B, Liang SX (2022) New insight into the co-adsorption of oxytetracycline and Pb (II) using magnetic metal–organic frameworks composites in aqueous environment: co-adsorption mechanisms and application potentials. Environ Sci Pollut R. https://doi.org/10.1007/s11356-022-19339-z
Wu W, Chen D, Li J, Su M, Chen N (2018) Enhanced adsorption of uranium by modified red muds: adsorption behavior study. Environ Sci Pollut R 25:18096–18108. https://doi.org/10.1007/s11356-018-2027-x
Xu H, Zhu S, Xia M, Wang F (2021) Rapid and efficient removal of diclofenac sodium from aqueous solution via ternary core-shell CS@ PANI@ LDH composite: experimental and adsorption mechanism study. J Hazard Mater 402:123815. https://doi.org/10.1016/j.jhazmat.2020.123815
Yan Y, Chu Y, Khan MA, Xia M, Shi M, Zhu S, Lei W, Wang F (2022) Facile immobilization of ethylenediamine tetramethylene-phosphonic acid into UiO-66 for toxic divalent heavy metal ions removal: an experimental and theoretical exploration. Sci Total Environ 806:150652. https://doi.org/10.1016/j.scitotenv.2021.150652
Zaichik S, Steinbring C, Jelkmann M, Bernkop-Schnurch A (2020) Zeta potential changing nanoemulsions: impact of PEG-corona on phosphate cleavage. Int J Pharm 581:119299. https://doi.org/10.1016/j.ijpharm.2020.119299
Zhang XJ, Ma TY, Yuan ZY (2008) Synthesis of hierarchically meso-/macroporous titanium tetraphosphonate materials with large adsorption capacity of heavy metal ions. J Phys Chem Lett 37:746–747. https://doi.org/10.1246/cl.2008.746
Zhang Y, Xu Q, Zhang S, Liu J, Zhou J, Xu H, Xiao H, Li J (2013) Preparation of thiol-modified Fe3O4@SiO2 nanoparticles and their application for gold recovery from dilute solution. Sep Purif 116:391–397. https://doi.org/10.1016/j.seppur.2013.06.018
Zheng J, Liu ZQ, Zhao XS, Liu M, Liu X, Chu W (2012) One-step solvothermal synthesis of Fe3O4@C core-shell nanoparticles with tunable sizes. Nanotechnology 23:165601. https://doi.org/10.1088/0957-4484/23/16/165601
Zhou Y, Li Y, Wang X, Liu D, Liu D (2020) Preparation of amidoxime functionalized titanate nanosheets for efficient extraction of uranium from aqueous solution. J Solid State Chem 290:121562. https://doi.org/10.1016/j.jssc.2020.121562
Zhu S, Khan MA, Kameda T, Xu H, Wang F, Xia M, Yoshioka T (2022) New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J Hazard Mater 426:128062. https://doi.org/10.1016/j.jhazmat.2021.128062
Zong E, Wei D, Wan H, Zheng S, Xu Z, Zhu D (2013) Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem Eng J 221:193–203. https://doi.org/10.1016/j.cej.2013.01.088
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21906017, 21866004, 21866003) and Jiangxi Provincial Natural Science Foundation (grant nos. 20202BABL213026 and 20202BABL203016).
Availability of data and materials
All data generated or analyzed during this study are included in the manuscript.
Author information
Authors and Affiliations
Contributions
Qie Luo wrote the entire draft of the manuscript. The core conceptual idea and study design were all provided by Youqun Wang and Xiaohong Cao separately. The preparation and adsorptive experiments were conducted with Bo Xiao. Material preparation and data analysis were completed by WenZheng Cui, Huan Wang, and Yunhai Liu. The characterization analysis of the adsorbents was worked out by Zhibin Zhang and Lei Chen.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
The authors have consent to participate.
Consent for publication
The authors have consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Georg Steinhauser
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1582 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Q., Cui, W., Wang, H. et al. Efficient capture of U(VI) by magnetic Zr(IV)-ethylenediamine tetramethylene phosphonic acid inorganic-organic hybrid. Environ Sci Pollut Res 29, 68320–68331 (2022). https://doi.org/10.1007/s11356-022-20548-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20548-9