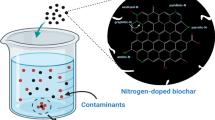
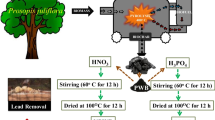
Abstract
The surface properties of the adsorbents and the acidic environment have an influence effect on Pb adsorption. In order to further improve the adsorption performance of biochar, we herein reported an effective method to synthesize high-adsorbed biochar by co-doping with nitrogen and phosphorus. After atom doping, the N/P co-doped biochar (NP-BC) showed the enhanced adsorption capacity for lead ion (Pb2+). The adsorption kinetics, isotherm, pH value, and influencing factors were studied. The results show that the synthesized NP-BC has high Pb2+ adsorption capacity in aqueous solution, and can be maintained with various environmental interference factors including pH, natural organic matter, and other metal ions. High adsorption performance shows that the material may be well used to remove Pb2+ in various water bodies. Various characterization experiments prove that surface properties contribute to Pb2+ adsorption, and the high performance of NP-BC is mainly due to the surface complexation between functional groups and Pb2+. This work demonstrates that the surface functional groups of biochar are critical to the development of high-performance heavy metal adsorbents.
Graphical abstract







Similar content being viewed by others
References
Ahrouch M, Gatica JM, Draoui K, Bellido D, Vidal H (2019) Lead removal from aqueous solution by means of integral natural clays honeycomb monoliths. J. Hazard. Mater 365:519–530. https://doi.org/10.1016/j.jhazmat.2018.11.037
Benhaddya ML, Halis Y, Lahcini A (2019) Concentration, distribution, and potential aquatic risk assessment of metals in water from Chott Merouane (Ramsar Site), Algeria. Arch Environ Contam Toxicol 77:127–143. https://doi.org/10.1007/s00244-019-00631-y
Bolisetty S, Peydayesh M, Mezzenga R (2019) Sustainable technologies for water purification from heavy metals: review and analysis. Chem. Soc. Rev. 48:463–487. https://doi.org/10.1039/C8CS00493E
Chen M, He F, Hu D, Bao C, Huang Q (2020) Broadened operating pH range for adsorption/reduction of aqueous Cr(VI) using biochar from directly treated jute (Corchorus capsularis L.) fibers by H3PO4. Chem. Eng 381:122739. https://doi.org/10.1016/j.cej.2019.122739
Chen M, Li F, Tao M, Hu L, Shi Y, Liu Y (2019) Distribution and ecological risks of heavy metals in river sediments and overlying water in typical mining areas of China. Mar. Pollut. Bull 146:893–899. https://doi.org/10.1016/j.marpolbul.2019.07.029
Chu G, Zhao J, Huang Y et al (2018) Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 240:1–9. https://doi.org/10.1016/j.envpol.2018.04.003
Goyal N, Gao P, Wang Z et al (2020) Nanostructured chitosan/molecular sieve-4A an emergent material for the synergistic adsorption of radioactive major pollutants cesium and strontium. J. Hazard. Mater 392:122494. https://doi.org/10.1016/j.jhazmat.2020.122494
Guo R, Yan L, Rao P, Wang R, Guo X (2020) Nitrogen and sulfur co-doped biochar derived from peanut shell with enhanced adsorption capacity for diethyl phthalate. Environ. Pollut. 258:113674. https://doi.org/10.1016/j.envpol.2019.113674
Guo Y, Rockstraw DA (2007) Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation. Bioresour. Technol 98:1513–1521. https://doi.org/10.1016/j.biortech.2006.06.027
Guo Z, Zhang J, Liu H, Kang Y (2017) Development of a nitrogen-functionalized carbon adsorbent derived from biomass waste by diammonium hydrogen phosphate activation for Cr(VI) removal. Powder Technol 318:459–464. https://doi.org/10.1016/j.powtec.2017.06.024
Jiang C, Liu Y, Yuan D, Wang Y, Liu J, Chew JW (2020) Investigation of the high U(VI) adsorption properties of phosphoric acid-functionalized heteroatoms-doped carbon materials. Solid State Sci. 104:106248. https://doi.org/10.1016/j.solidstatesciences.2020.106248
Kushwaha A, Rani R, Patra JK (2020) Adsorption kinetics and molecular interactions of lead [Pb(II)] with natural clay and humic acid. Int J Environ SCI TE 17:1325–1336. https://doi.org/10.1007/s13762-019-02411-6
Li L, Zou D, Xiao Z et al (2019a) Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J. Clean. Prod 210:1324–1342. https://doi.org/10.1016/j.jclepro.2018.11.087
Li X, Liu J, Gong X, Qing T, Zhang P, Feng B (2019b) Synthesis of fluorescent tungsten disulfide by nitrogen atom doping and its application for mercury(ii) detection. J. Mater. Chem. C 7:4096–4101. https://doi.org/10.1039/C8TC06233A
Lian F, Cui G, Liu Z, Duo L, Zhang G, Xing B (2016) One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J.Environ. Manage 176:61–68. https://doi.org/10.1016/j.jenvman.2016.03.043
Ling L-L, Liu W-J, Zhang S, Jiang H (2017) Magnesium oxide embedded nitrogen self-doped biochar composites: fast and high-efficiency adsorption of heavy metals in an aqueous solution. Environ. Sci. Technol 51:10081–10089. https://doi.org/10.1021/acs.est.7b02382
Liu S, Wu P, Chen M, et al., 2017. Amphoteric modified vermiculites as adsorbents for enhancing removal of organic pollutants: bisphenol A and Tetrabromobisphenol A. Environ. Pollut. 228, 277-286. https://doi.org/10.1016/j.envpol.2017.03.082.
Liu T, Lawluvy Y, Shi Y et al (2021) Adsorption of cadmium and lead from aqueous solution using modified biochar: a review. J Environ Chem Eng. 7:106502. https://doi.org/10.1016/j.jece.2021.106502
Lu M, Zhang Y, Zhou Y et al (2019) Adsorption-desorption characteristics and mechanisms of Pb(II) on natural vanadium, titanium-bearing magnetite-humic acid magnetic adsorbent. PowderTechnol 344:947–958. https://doi.org/10.1021/acs.est.7b02382
Mahar A, Wang P, Ali A, et al. (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol. Environ. Saf.126, 111-121. 10.1016/j.ecoenv.2015.12.023
Ma H, Xu Z, Wang W et al (2019) Adsorption and regeneration of leaf-based biochar for p-nitrophenol adsorption from aqueous solution. RSC Adv. 67:39282–39293. https://doi.org/10.1039/c9ra07943b
Medina RP, Nadres ET, Ballesteros FC, Rodrigues DF (2016) Incorporation of graphene oxide into a chitosan–poly(acrylic acid) porous polymer nanocomposite for enhanced lead adsorption. Environ. Sci. Nano.638-646.doi: 10.1039/C6EN00021E.
Miao S, Liang K, Zhu J, Yang B, Zhao D, Kong B (2020) Hetero-atom-doped carbon dots: doping strategies, properties and applications. Nano Today.33, 100879.doi: 10.1016/j.nantod.2020.100879.
Peng H, Gao P, Chu G, Pan B, Peng J, Xing B (2017) Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut.229, 846-853. 10.1016/j.envpol.2017.07.004.
Rangabhashiyam S, Balasubramanian P (2019) Characteristics, performances, equilibrium and kinetic modeling aspects of heavy metal removal using algae. Bioresour. Technol. Rep.5, 261-279. 10.1016/j.biteb.2018.07.009.
Tan X, Liu Y, Zeng G et al (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere. 125:70–85. https://doi.org/10.1016/j.chemosphere.2014.12.058
Wang H, Cai J, Liao Z, et al. (2020) Black liquor as biomass feedstock to prepare zero-valent iron embedded biochar with red mud for Cr(VI) removal: mechanisms insights and engineering practicality. Bioresour. Technol.311, 123553. 10.1016/j.biortech.2020.123553.
Wang L, Wang Y, Ma F, et al. (2019) Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: a review. Sci. Total Environ.668, 1298-1309. 10.1016/j.scitotenv.2019.03.011
Wu J, Wang T, Zhang Y, Pan W-P (2019) The distribution of Pb(II)/Cd(II) adsorption mechanisms on biochars from aqueous solution: considering the increased oxygen functional groups by HCl treatment. Bioresour. Technol.291, 121859. 10.1016/j.biortech.2019.121859
Xie Y, Yuan X, Wu Z, et al. (2019) Adsorption behavior and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon spheres on the removal of Pb(II) and Cu(II). J.ColloidInterfaceSci.536, 440-455. 10.1016/j.jcis.2018.10.066.
Xu X, Zheng Y, Gao B, Cao X (2019) N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chem. Eng.J.368, 564-572. 10.1016/j.cej.2019.02.165
Yang S, Zhao D, Zhang H, Lu S, Chen L, Yu X (2010) Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. J.Hazard. Mater.18, 632-640. 10.1016/j.jhazmat.2010.07.072.
Zeng Q, Huang Y, Huang L et al (2020) High adsorption capacity and super selectivity for Pb(II) by a novel adsorbent: nano humboldtine/almandine composite prepared from natural almandine. Chemosphere. 253:126650. https://doi.org/10.1016/j.chemosphere.2020.126650
Zhang H, Wen J, Fang Y, Zhang S, Zeng G (2019) Influence of fulvic acid on Pb(II) removal from water using a post-synthetically modified MIL-100(Fe). J. Colloid Interface Sci.551, 155-163. 10.1016/j.jcis.2019.05.016.
Zhang J, Dai L (2016) Nitrogen, phosphorus, and fluorine tri-doped graphene as a multifunctional catalyst for self-powered electrochemical water splitting. Angew. Chem. Int. Ed.55, 13296-13300. 10.1002/anie.201607405.
Zhao M, Dai Y, Zhang M, et al. (2020) Mechanisms of Pb and/or Zn adsorption by different biochars: biochar characteristics, stability, and binding energies. Sci. Total Environ.717, 136894. 10.1016/j.scitotenv.2020.136894.
Zhou N, Chen H, Feng Q, et al. (2017) Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod.165, 221-230. 10.1016/j.jclepro.2017.07.111.
Acknowledgements
Xiangtan University School of Environment and Resources, University of Science and Technology of China.
Availability of data and materials
All the data used for this work are publicly available.
Funding
This work was supported by the Xiangtan University College of Environment and Resources (2017SK2323), Hunan Environmental Protection and Air Pollution Control Engineering Technology Center (2018GK4021), National Natural Science Foundation of China (2018112500105), and Hunan Provincial Science and Technology Department Project (2018112500409).
Author information
Authors and Affiliations
Contributions
Pan Jing: conceptualization, investigation, data curation, writing - original draft; Deng Haowang: data curation, writing - review and editing; Du Ziyan: data curation, writing - review and editing; Tian Ke: resources, supervision, project administration, funding acquisition; Zhang Junfeng: supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors consent to publish this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1824 kb)
Rights and permissions
About this article
Cite this article
Pan, J., Deng, H., Du, Z. et al. Design of nitrogen-phosphorus-doped biochar and its lead adsorption performance. Environ Sci Pollut Res 29, 28984–28994 (2022). https://doi.org/10.1007/s11356-021-17335-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17335-3