Abstract
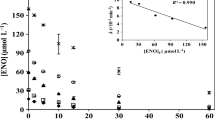
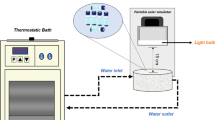
Polychlorinated biphenyls (PCBs) are a family of highly toxic, resistant, and persistent organic pollutants, among which 2-chlorobiphenyl (PCB-1) is one of the simplest. Most studies on PCBs’ photochemistry are limited to their direct photolysis, while the important role of reactive photo-induced species (RPS) (hydroxyl radicals, HO●; singlet oxygen, 1O2; and triplet excited states of chromophoric dissolved organic matter, 3CDOM*) in removing PCBs in natural waters through indirect photolysis has not yet been evaluated. In this work, the rate constants of the reactions between aqueous PCB-1 and RPS were obtained under simulated solar radiation (450-W Xenon lamp and an AM 1.5 global filter) by competition kinetics, and the effects of the initial pollutant concentration and the physicochemical characteristics of the water were investigated. The direct photolysis quantum yield of PCB-1 in the range 290–800 nm was found as 1.60 × 10−2 mol Einstein−1. The value of kPCB-1,HO● = (6.80 ± 0.09) × 109 L mol−1 s−1 is in good agreement with the literature. For 1O2, kPCB-1,1O2 = (1.13 ± 0.20) × 106 L mol−1 s−1, while for 3CDOM*, kPCB-1,3CBBP* = (2.44 ± 0.04) × 109 L mol−1 s−1 and kPCB-1,3AQ2S* = (3.36 ± 0.04) × 109 L mol−1 s−1 were obtained using 4-benzoylbenzoic acid (CBBP) and anthraquinone-2-sulfonate (AQ2S) as CDOM proxies, respectively. These results show that the main pathways involved in PCB-1 photodegradation are the reactions with HO● and 3CDOM* together with direct photolysis. In addition, the photodegradation of PCB-1 in sunlit waters was simulated using the kinetic model APEX (Aqueous Photochemistry of Environmentally Occurring Xenobiotics). According to simulations, a greater influence of the water depth and dissolved organic carbon concentration (DOC) on the persistence of PCB-1 is expected, being only slightly influenced by the concentrations of nitrite, nitrate, and bicarbonate. Finally, based on data reported for Brazilian surface waters, the average half-life (t1/2) of PCB-1 is expected to vary from 2 to 14 days. In particular, the t1/2 in the Paranapanema River is estimated at 7 to 8 days.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
ABNT - Associação Brasileira de Normas Técnicas (1992a) ABNT NBR 12619:1992 Versão Corrigida:1995. Waters - Determination of nitrite-sulfanilamide and N-(1-naftil) - ethylenediamine methods - Method of test. Rio de Janeiro
ABNT - Associação Brasileira de Normas Técnicas (1992b) ABNT NBR 12620:1992. Waters - Determination of nitrate - Chromotropic acid and phenoldissulfonic acid methods - Method of test. Rio de Janeiro
ABNT - Associação Brasileira de Normas Técnicas (1996) ABNT NBR 13736:1996. Water - Alkalinity determination potenciometric and titulometric methods - Method of test. Rio de Janeiro
al Housari F, Vione D, Chiron S, Barbati S (2010) Reactive photoinduced species in estuarine waters. Characterization of hydroxyl radical, singlet oxygen and dissolved organic matter triplet state in natural oxidation processes. Photochem Photobiol Sci 9:78–86
Alansi AM, Qahtan TF, Saleh TA (2021) Solar-driven fixation of bismuth oxyhalides on reduced graphene oxide for efficient sunlight-responsive immobilized photocatalytic systems. Adv Mater Interfaces 8:2001463
Araujo LG, Santos FS, Teixeira ACSC (2017) Degradation of bisphenol A by the UV and UV/H2O2 processes: Evaluation of process variables through experimental design. Can J Chem Eng 95:2278–2285
Avetta P, Fabbri D, Minella M, Brigante M, Maurino V, Minero C, Pazzi M, Vione D (2016) Assessing the phototransformation of diclofenac, clofibric acid and naproxen in surface waters: model predictions and comparison with field data. Water Res 105:383–394
Bao YP, Huang QY, Wang WL, Xu JJ, Jiang F, Feng CH (2011) Application of quantum chemical descriptors into quantitative structure-property relationship models for prediction of the photolysis half-life of PCBs in water. Front Environ Sci Eng China 5:505–511
Basaleh AA, Al-Malack MH, Saleh TA (2020) Polyamide-baghouse dust nanocomposite for removal of methylene blue and metals: Characterization, kinetic, thermodynamic and regeneration. Chinese J Chem Eng. https://doi.org/10.1016/j.cjche.2020.08.050
Basaleh AA, Al-Malack MH, Saleh TA (2021) Poly(acrylamide acrylic acid) grafted on steel slag as an efficient magnetic adsorbent for cationic and anionic dyes. J Environ Chem Eng 9:105126
Carena L, Puscasu CG, Comis S, Sarakha M, Vione D (2019) Environmental photodegradation of emerging contaminants: A re-examination of the importance of triplet-sensitised processes, based on the use of 4-carboxybenzophenone as proxy for the chromophoric dissolved organic matter. Chemosphere 237:124476
Chang FC, Chiu TC, Yen JH, Wang YS (2003a) Dechlorination pathways of ortho-substituted PCBs by UV irradiation in n-hexane and their correlation to the charge distribution on carbon atom. Chemosphere 51:775–784
Chang FC, Hsieh YN, Wang YS (2003b) Dechlorination of PCBs in water under UV irradiation and the relationship between the electric charge distribution on the carbon atom and the site of dechlorination occurrence. B Environ Contam Tox 71:971–978
Chen L, Tang XJ, Shen CF, Chen C, Chen YX (2012) Photosensitized degradation of 2,4’,5-trichlorobiphenyl (PCB 31) by dissolved organic matter. J Hazard Mater 201:1–6
Dadkhah H, Behnajady MA (2017) Photooxidative removal of p-nitrophenol by UV/H2O2 process in a spinning disk photoreactor: Influence of operating parameters. Chem Biochem Eng Q 31(3):361–368
Dudasova H, Derco J, Sumegova L, Dercova K, Laszlova K (2017) Removal of polychlorinated biphenyl congeners in mixture Delor 103 from wastewater by ozonation vs/and biological method. J Hazard Mater 321:54–61
Elovitz MS, von Gunten U (1999) Hydroxyl radical ozone ratios during ozonation processes. I-the R-Ct Concept Ozone-Sci Eng 21:239–260
Fabbri D, Minella M, Maurino V, Minero C, Vione D (2015) A model assessment of the importance of direct photolysis in the photo-fate of cephalosporins in surface waters: Possible formation of toxic intermediates. Chemosphere 134:452–458
Giokas DL, Vlessidis AG (2007) Application of a novel chemometric approach to the determination of aqueous photolysis rates of organic compounds in natural waters. Talanta 71:288–295
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interf Sci 309:464–469
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng C 32:12–17
Hawari J, Demeter A, Samson R (1992) Sensitized photolysis of polychlorobiphenyls in alkaline 2-propanol - dechlorination of aroclor 1254 in soil samples by solar-radiation. Environ Sci Technol 26:2022–2027
Huang IW, Hong CS, Bush B (1996) Photocatalytic degradation of PCBs in TiO2 aqueous suspensions. Chemosphere 32:1869–1881
Huang L, Dong WB, Hou HQ (2013) Photochemical reaction of 2-chlorobiphenyl with N(III) (H2ONO+/HONO/NO2-) in acidic environment studied by using co-linear laser flash photolysis. J Photoch Photobio A 268:44–49
Jain R, Mathur M, Sikarwar S, Mittal A (2007) Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J Environ Manage 85:956–964
Lastre-Acosta AM, Cristofoli BS, Parizi MPS, do Nascimento CAO, Teixeira A (2021) Photochemical persistence of sulfa drugs in aqueous medium: kinetic study and mathematical simulations. Environ Sci Pollut R 28:23887–23895
Lepine FL, Milot SM, Vincent NM, Gravel D (1991) Photochemistry of higher chlorinated pcbs in cyclohexane. J Agr Food Chem 39:2053–2056
Lores M, Llompart M, Gonzalez-Garcia R, Gonzalez-Barreiro C, Cela R (2002) Photolysis of polychlorinated biphenyls by solid-phase microextraction - “On-fibre” versus aqueous photodegradation. J Chromatogr A 963:37–47
Miao XS, Chu SG, Xu XB (1999) Degradation pathways of PCBs upon UV irradiation in hexane. Chemosphere 39:1639–1650
Moore T, Pagni RM (1987) Unusual photochemistry of 4-chlorobiphenyl in water. J Org Chem 52:770–773
Mostafa S, Rosario-Ortiz FL (2013) Singlet oxygen formation from wastewater organic matter. Environ Sci Technol 47:8179–8186
Passananti M, Temussi F, Iesce MR, Previtera L, Mailhot G, Vione D, Brigante M (2014) Photoenhanced transformation of nicotine in aquatic environments: involvement of naturally occurring radical sources. Water Res 55:106–114
Reddy AVB, Moniruzzaman M, Aminabhavi TM (2019) Polychlorinated biphenyls (PCBs) in the environment: recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem Eng J 358:1186–1207
Saleh TA, Ali I (2018) Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals. J Environ Chem Eng 6:5361–5368
Schwarzenbach RP (2003) Environmental organic chemistry, 2nd edn. Wiley & Sons Inc, Hoboken
Shemer H, Sharpless CM, Elovitz MS, Linden KG (2006) Relative rate constants of contaminant candidate list pesticides with hydroxyl radicals. Environ Sci Technol 40:4460–4466
Silva MP, Mostafa S, McKay G, Rosario-Ortiz FL, Teixeira A (2015) Photochemical fate of amicarbazone in aqueous media: laboratory measurement and simulations. Environ Eng Sci 32:730–740
Vetter W, Luckas B, Buijten J (1998) Elution order of the 209 polychlorinated biphenyls on a high-temperature capillary column. J Chromatogr A 799:249–258
Vione D, Encinas A, Fabbri D, Calza P (2018a) A model assessment of the potential of river water to induce the photochemical attenuation of pharmaceuticals downstream of a wastewater treatment plant (Guadiana River, Badajoz, Spain). Chemosphere 198:473–481
Vione D, Fabbri D, Minella M, Canonica S (2018b) Effects of the antioxidant moieties of dissolved organic matter on triplet-sensitized phototransformation processes: implications for the photochemical modeling of sulfadiazine. Water Res 128:38–48
Vione D (2020) A critical view of the application of the APEX software (Aqueous Photochemistry of Environmentally-Occurring Xenobiotics) to Predict Photoreaction Kinetics in Surface Freshwaters. Molecules 25:9
Vione D, Minella M, Maurino V, Minero C (2014) Indirect photochemistry in sunlit surface waters: Photoinduced production of reactive transient species. Chem Eur J 20:10590–10606
Acknowledgements
This work was financially supported by CAPES. Finance Code 001 and post-doc grant #88887.340964/2019-00. The authors are grateful to the National Council for Scientific and Technological Development – Brazil (CNPq, grant #311230/2020-2) and to the São Paulo Research Foundation (FAPESP) (grant #2019/24158-9). The authors thank Prof. Marcela Prado Silva Parizi and undergraduate students Gabriela de Souza Freitas and Ana Caroline Fernandes Borges (São Paulo State University, Campus Rosana) for carrying out the analysis of natural water samples from the Paranapanema River.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES)—Finance Code 001 and post-doc grant #88887.340964/2019–00.
National Council for Scientific and Technological Development (CNPq)—grant #311230/2020–2.
São Paulo Research Foundation (FAPESP)—grant #2019/24158–9.
Author information
Authors and Affiliations
Contributions
AMLA: conceptualization, methodology, validation, formal analysis, investigation, writing (original draft), visualization.
CMR: methodology, validation, investigation.
MAM: methodology, investigation, writing (review and editing), supervision.
ACSCT: conceptualization, validation, writing (original draft), writing (review and editing), supervision, funding acquisition.
CAON: resources, supervision, funding acquisition, writing—review and editing.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lastre-Acosta, A.M., Rocha, C.M., Mendes, M.A. et al. Sunlight-driven environmental photodegradation of 2-chlorobiphenyl (PCB-1) in surface waters: kinetic study and mathematical simulations. Environ Sci Pollut Res 29, 42231–42241 (2022). https://doi.org/10.1007/s11356-021-17010-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17010-7