Abstract
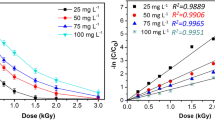
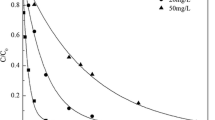
Pharmaceutical compounds were emerging contaminants, and the accumulation of pharmaceutical compounds in the environment increased the risk to humans and ecosystems. In this study, electron beam irradiation was applied to degrade indomethacin (IDM) in aqueous solution. IDM degradation followed pseudo-first-order kinetics and 300 μM IDM could be completely degraded at only 2 kGy. According to the quenching experiment, the dose constant ratios of oxidative radicals (•OH) and reductive radicals (e−aq and •H) could be calculated as k•OH: ke aq and •H=4.79:1. As the concentration of H2O2 increased from 0 to 10 mM, the dose constant increased from 1.883 to 2.582 kGy−1. However, degradation effect would be restrained in the existence of NO−3, NO−2, CO2−3, HCO−3, SO2−, and humic acid due to their competition for the active species. Theoretical calculation revealed the radical attacking sites of IDM molecule and the most probable pathways were proposed with identification of intermediates. The attack of •OH mainly resulted in the cleavage of amide bond, indole ring opening, demethoxylation, and •OH addition. Dechlorination and the reduction of the carbonyl group occurred on IDM molecular through the reduction of e−aq and •H. The intermediates could continue to be degraded to small molecule acid, such as formic acid, acetic acid, and oxalic acid. Furthermore, highly toxic IDM transformed into less toxic products during the irradiation process.








Similar content being viewed by others
Data availability
All date generated or analyzed during this study are included in this article and its supplementary information files.
References
Abdel Daiem MM, Rivera-Utrilla J, Ocampo-Pérez R, Sánchez-Polo M, López-Peñalver JJ (2013) Treatment of water contaminated with diphenolic acid by gamma radiation in the presence of different compounds. Chemical Engineering Journal 219:371–379
An T, Yang H, Li G, Song W, Cooper WJ, Nie X (2010) Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Applied Catalysis B: Environmental 94:288–294
An T, Gao Y, Li G, Kamat PV, Peller J, Joyce MV (2014) Kinetics and mechanism of •OH mediated degradation of dimethyl phthalate in aqueous solution: experimental and theoretical studies. Environmental Science & Technology 48:641–648
Archer E, Petrie B, Kasprzyk-Hordern B, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174:437–446
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in Aqueous Solution). Journal of Physical and Chemical Reference Data 17:513–886
Changotra R, Guin JP, Khader SA, Varshney L, Dhir A (2019) Electron beam induced degradation of ofloxacin in aqueous solution: kinetics, removal mechanism and cytotoxicity assessment. Chemical Engineering Journal 356:973–984
Chen J, Qu R, Pan X, Wang Z (2016) Oxidative degradation of triclosan by potassium permanganate: kinetics, degradation products, reaction mechanism, and toxicity evaluation. Water Research 103:215–223
Chen J, Xu X, Pan X, Yao J, Li C, Qu R, Wang Z (2018) Mechanism insights into the oxidative degradation of decabromodiphenyl ethane by potassium permanganate in acidic conditions. Chemical Engineering Journal 332:267–276
De Vleeschouwer F, Van Speybroeck V, Waroquier M, Geerlings P, De Proft F (2007) Electrophilicity and nucleophilicity index for radicals. Organic Letters 9:2721–2724
Del Castillo I, Hernández P, Lafuente A, Rodríguez-Llorente ID, Caviedes MA, Pajuelo E (2012) Self-bioremediation of cork-processing wastewaters by (chloro)phenol-degrading bacteria immobilised onto residual cork particles. Water Research 46:1723–1734
Fukui K, Yonezawa T, Shingu H (1952) A Molecular orbital theory of reactivity in aromatic hydrocarbons. The Journal of Chemical Physics 20:722–725
Fukui K, Yonezawa T, Nagata C, Shingu H (1954) Molecular orbital theory of orientation in aromatic, heteroaromatic, and other conjugated molecules. The Journal of Chemical Physics 22:1433–1442
Huang J, Wang Y, Liu G, Chen P, Wang F, Ma J, Li F, Liu H, Lv W (2017) Oxidation of indometacin by ferrate (VI): kinetics, degradation pathways, and toxicity assessment. Environmental Science and Pollution Research 24:10786–10795
Jiménez JJ, Sánchez MI, Pardo R, Muñoz BE (2017) Degradation of indomethacin in river water under stress and non-stress laboratory conditions: degradation products, long-term evolution and adsorption to sediment. Journal of Environmental Sciences 51:13–20
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Research 42:3498–3518
Li WC (2014) Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environmental Pollution 187:193–201
Li M, Conrad B, Maus RG, Pitzenberger SM, Subramanian R, Fang X, Kinzer JA, Perpall HJ (2005) A novel oxidative degradation pathway of indomethacin under the stressing by hydrogen peroxide. Tetrahedron Letters 46:3533–3536
Li R, Kong J, Liu H, Chen P, Liu G, Li F, Lv W (2017) A sulfate radical based ferrous-peroxydisulfate oxidative system for indomethacin degradation in aqueous solutions. RSC Advances 7:22802–22809
Li F, Du P, Liu W, Li X, Ji H, Duan J, Zhao D (2018a) Hydrothermal synthesis of graphene grafted titania/titanate nanosheets for photocatalytic degradation of 4-chlorophenol: solar-light-driven photocatalytic activity and computational chemistry analysis. Chemical Engineering Journal 331:685–694
Li R, Kong J, Liu H, Chen P, Su Y, Liu G, Lv W (2018b) Removal of indomethacin using UV–vis/peroxydisulfate: kinetics, toxicity, and transformation pathways. Chemical Engineering Journal 331:809–817
Lian L, Yao B, Hou S, Fang J, Yan S, Song W (2017) Kinetic study of hydroxyl and sulfate radical-mediated oxidation of pharmaceuticals in wastewater effluents. Environmental Science & Technology 51:2954–2962
Lin T, Yu S, Chen W (2016) Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 152:1–9
Liu Y, Wang J (2013) Degradation of sulfamethazine by gamma irradiation in the presence of hydrogen peroxide. J Hazard Mater 250-251:99–105
Liu N, Wang T, Zheng M, Lei J, Tang L, Hu G, Xu G, Wu M (2015a) Radiation induced degradation of antiepileptic drug primidone in aqueous solution. Chemical Engineering Journal 270:66–72
Liu X, Zhang T, Wang L, Shao Y, Fang L (2015b) Hydrated electron-based degradation of atenolol in aqueous solution. Chemical Engineering Journal 260:740–748
Liu N, Lei Z-D, Wang T, Wang J-J, Zhang X-D, Xu G, Tang L (2016) Radiolysis of carbamazepine aqueous solution using electron beam irradiation combining with hydrogen peroxide: Efficiency and mechanism. Chemical Engineering Journal 295:484–493
Liu H, Yao J, Wang L, Wang X, Qu R, Wang Z (2019) Effective degradation of fenitrothion by zero-valent iron powder (Fe0) activated persulfate in aqueous solution: kinetic study and product identification. Chemical Engineering Journal 358:1479–1488
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. Journal of Computational Chemistry 33:580–592
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Science of The Total Environment 473-474:619–641
Lyu G, Shi G, Tang L, Fang H, Wu M (2017) Mechanism of degradation of a nitrogenous heterocycle induced by a reductive radical: decomposition of a sym-triazine ring. Physical Chemistry Chemical Physics 19:9354–9357
Oláh J, Van Alsenoy C, Sannigrahi AB (2002) Condensed fukui functions derived from stockholder charges: assessment of their performance as local reactivity descriptors. The Journal of Physical Chemistry A 106:3885–3890
Panorel I, Preis S, Kornev I, Hatakka H, Louhi-Kultanen M (2013) Oxidation of aqueous pharmaceuticals by pulsed corona discharge. Environmental Technology 34:923–930
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. Journal of the American Chemical Society 106:4049–4050
Rivera-Jaimes JA, Postigo C, Melgoza-Alemán RM, Aceña J, Barceló D, López de Alda M (2018) Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Science of The Total Environment 613-614:1263–1274
Rosal R, Rodríguez A, Perdigón-Melón JA, Petre A, García-Calvo E, Gómez MJ, Agüera A, Fernández-Alba AR (2010) Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Research 44:578–588
Sánchez-Polo M, López-Peñalver J, Prados-Joya G, Ferro-García MA, Rivera-Utrilla J (2009) Gamma irradiation of pharmaceutical compounds, nitroimidazoles, as a new alternative for water treatment. Water Research 43:4028–4036
Scholz M, Blobaum AL, Marnett LJ, Hey-Hawkins E (2012) Ortho-carbaborane derivatives of indomethacin as cyclooxygenase (COX)-2 selective inhibitors. Bioorganic & medicinal chemistry 20:4830–4837
Secretariat UNECfE (2009) Globally harmonized system of classification and labelling of chemicals (GHS). United Nations Publications
Shao H-Y, Wu M-h, Deng F, Xu G, Liu N, Li X, Tang L (2018) Electron beam irradiation induced degradation of antidepressant drug fluoxetine in water matrices. Chemosphere 190:184–190
Subedi B, Du B, Chambliss CK, Koschorreck J, Rüdel H, Quack M, Brooks BW, Usenko S (2012) Occurrence of pharmaceuticals and personal care products in german fish tissue: A National Study. Environmental Science & Technology 46:9047–9054
Sui Q, Huang J, Deng S, Yu G, Fan Q (2010) Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Research 44:417–426
Tanoue R, Nomiyama K, Nakamura H, Kim J-W, Isobe T, Shinohara R, Kunisue T, Tanabe S (2015) Uptake and tissue distribution of pharmaceuticals and personal care products in wild fish from treated-wastewater-impacted streams. Environmental Science & Technology 49:11649–11658
Wang J, Chu L (2016) Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: an overview. Radiation Physics and Chemistry 125:56–64
Wang J, Wang S (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. Journal of environmental management 182:620–640
Wang F, Chen P, Feng Y, Xie Z, Liu Y, Su Y, Zhang Q, Wang Y, Yao K, Lv W, Liu G (2017) Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Applied Catalysis B: Environmental 207:103–113
Xie H, Hao H, Xu N, Liang X, Gao D, Xu Y, Gao Y, Tao H, Wong M (2019) Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: occurrence, distribution, potential sources, and health risk assessment. Science of The Total Environment 659:230–239
Xu G, Yao J-z, Tang L, Yang X-y, Zheng M, Wang H, Wu M-h (2015) Electron beam induced degradation of atrazine in aqueous solution. Chemical Engineering Journal 275:374–380
Xu Z, Zhang X, Huang N, Hu H-y (2017) Oxidation of benzalkonium chloride by gamma irradiation: kinetics and decrease in toxicity. Journal of Radioanalytical and Nuclear Chemistry 312:631–637
Yang W, Mortier WJ (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. Journal of the American Chemical Society 108:5708–5711
Yang W, Parr RG (1985) Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proceedings of the National Academy of Sciences 82:6723–6726
Yang Y, Ok YS, Kim K-H, Kwon EE, Tsang YF (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Science of The Total Environment 596-597:303–320
Yu S, Lee B, Lee M, Cho I-H, Chang S-W (2008) Decomposition and mineralization of cefaclor by ionizing radiation: kinetics and effects of the radical scavengers. Chemosphere 71:2106–2112
Zhang S-J, Jiang H, Li M-J, Yu H-Q, Yin H, Li Q-R (2007) Kinetics and mechanisms of radiolytic degradation of nitrobenzene in aqueous solutions. Environmental Science & Technology 41:1977–1982
Zhang Q, Chen P, Zhuo M, Wang F, Su Y, Chen T, Yao K, Cai Z, Lv W, Liu G (2018) Degradation of indometacin by simulated sunlight activated CDs-loaded BiPO4 photocatalyst: roles of oxidative species. Applied Catalysis B: Environmental 221:129–139
Zhao Y, Kuang J, Zhang S, Li X, Wang B, Huang J, Deng S, Wang Y, Yu G (2017) Ozonation of indomethacin: kinetics, mechanisms and toxicity. Journal of Hazardous Materials 323:460–470
Zheng BG, Zheng Z, Zhang JB, Luo XZ, Wang JQ, Liu Q, Wang LH (2011) Degradation of the emerging contaminant ibuprofen in aqueous solution by gamma irradiation. Desalination 276:379–385
Zhou J, Broodbank N (2014) Sediment-water interactions of pharmaceutical residues in the river environment. Water Research 48:61–70
Zhou JL, Zhang ZL, Banks E, Grover D, Jiang JQ (2009) Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. Journal of Hazardous Materials 166:655–661
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 11975147, 12075148, 12075146, and 41773121), National Key Research and Development Project (No. 2020YFC1808200), and Project supported by Science and Technology Commission of Shanghai Municipality (20010500300).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yu Duan: data curation, writing-original draft preparation. Wei Zhou: investigation, writing-reviewing and editing. Haiyang Shao: conceptualization, investigation. Zhibo Zhang: investigation, validation. Wenyan Shi: methodology, investigation. Gang Xu: conceptualization, methodology. All authors read the approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Weiming Zhang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Duan, Y., Zhou, W., Shao, H. et al. Electron beam induced degradation of indomethacin in aqueous solution: kinetics, degradation mechanism, and toxicity assessment. Environ Sci Pollut Res 29, 19283–19294 (2022). https://doi.org/10.1007/s11356-021-16348-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16348-2