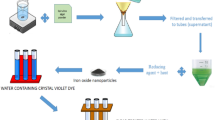
Abstract
Globally, organic dyes are major constituents in wastewater effluents due to their large-scale industrial applications. These persistent pollutants adversely impact the public health of different living entities. Thus, wastewater remediation has become an indispensable necessity. Herein, we greenly synthesized iron oxide nanoparticles (SP-IONPs) using Spirulina platensis microalgae to remove cationic crystal violet (CV) and anionic methyl orange (MO) dyes from their aqueous solution. The engineered sorbent was thoroughly scrutinized by different characterization techniques of FT-IR, BET surface area, SEM, EDX, TEM, VSM, UV/Vis spectroscopy, and pHPZC measurement. The proficiency of SP-IONPs was methodically appraised for its sorptive performance towards the target CV and MO dyes under variable technological parameters (batch scenario). Collectively, the outlined results inferred an amazing efficacy characterized to the SP-IONPs sorbent for the expulsion of relevant dyes from the aqueous media. Regarding the dynamic static sorption data, the kinetics profile was ascribed to the pseudo-second order model, whereas sorption isotherm was quantitatively dominated by the Langmuir theory with maximum sorption capacities of 256.4 mg g-1 and 270.2 mg g-1 for CV and MO, respectively. Thermodynamics findings conformed the endothermic nature of sorption process. Repeatability of the spent sorbent was successfully emphasized for 5 times of sorption/desorption cycles. The productive sorbent admirably sequestered CV and MO dyes from spiked tap water. The potency of SP-IONPs as color collecting material from real dyeing effluents was achieved.
Graphical abstract















Similar content being viewed by others
Data availability
All data generated or analyzed during this study were included in the submitted article. In addition, the datasets used or analyzed during the current study were available from the corresponding author on reasonable request.
References
Abdi M, Balagabri M, Karimi H et al (2020) Degradation of crystal violet (CV) from aqueous solutions using ozone, peroxone, electroperoxone, and electrolysis processes: a comparison study. Appl Water Sci 10:168. https://doi.org/10.1007/s13201-020-01252-w
Aksu Demirezen D, Yıldız YŞ, Demirezen Yılmaz D (2019) Amoxicillin degradation using green synthesized iron oxide nanoparticles: Kinetics and mechanism analysis. Environ Nanotechnol Monit Manag 11:100219. https://doi.org/10.1016/j.enmm.2019.100219
Anwer H, Mahmood A, Lee J et al (2019) Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res 12:955–972
Asghar MA, Zahir E, Shahid SM et al (2018) Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT Food Sci Technol 90:98–107. https://doi.org/10.1016/j.lwt.2017.12.009
Badawi AK, Zaher K (2021) Hybrid treatment system for real textile wastewater remediation based on coagulation/flocculation, adsorption and filtration processes: Performance and economic evaluation. J Water Process Eng 40:101963. https://doi.org/10.1016/j.jwpe.2021.101963
Bakht Shokouhi S, Dehghanzadeh R, Aslani H, Shahmahdi N (2020) Activated carbon catalyzed ozonation (ACCO) of Reactive Blue 194 azo dye in aqueous saline solution: Experimental parameters, kinetic and analysis of activated carbon properties. J Water Process Eng 35:101188. https://doi.org/10.1016/j.jwpe.2020.101188
Bhowmik M, Kanmani M, Debnath A, Saha B (2019) Sono-assisted rapid adsorption of anionic dye onto magnetic CaFe2O4/MnFe2O4 nanocomposite from aqua matrix. Powder Technol 354:496–504. https://doi.org/10.1016/j.powtec.2019.06.009
Bibi I, Nazar N, Ata S et al (2019) Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J Mater Res Technol 8:6115–6124. https://doi.org/10.1016/j.jmrt.2019.10.006
Bishnoi S, Kumar A, Selvaraj R (2018) Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater Res Bull 97:121–127. https://doi.org/10.1016/j.materresbull.2017.08.040
Carvalho SSF, Carvalho NMF (2017) Dye degradation by green heterogeneous Fenton catalysts prepared in presence of Camellia sinensis. J Environ Manag 187:82–88. https://doi.org/10.1016/j.jenvman.2016.11.032
Cechinel MAP, Mayer DA, Mazur LP et al (2018) Application of ecofriendly cation exchangers (Gracilaria caudata and Gracilaria cervicornis) for metal ions separation and recovery from a synthetic petrochemical wastewater: Batch and fixed bed studies. J Clean Prod 172:1928–1945. https://doi.org/10.1016/j.jclepro.2017.11.235
Chatterjee A, Jana AK, Basu JK (2021) Silica supported binary metal organic framework for removing organic dye involving combined effect of adsorption followed by photocatalytic degradation. Mater Res Bull 138:111227. https://doi.org/10.1016/j.materresbull.2021.111227
Chen B, Zhao H, Chen S et al (2019) A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem Eng J 356:69–80. https://doi.org/10.1016/j.cej.2018.08.222
Chien SH, Clayton WR (1980) Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci Soc Am J 44:265–268. https://doi.org/10.2136/sssaj1980.03615995004400020013x
De Lima Barizão AC, Silva MF, Andrade M et al (2020) Green synthesis of iron oxide nanoparticles for tartrazine and bordeaux red dye removal. J Environ Chem Eng 8:103618. https://doi.org/10.1016/j.jece.2019.103618
Deniz F, Kepekci RA (2016) Dye biosorption onto pistachio by-product: A green environmental engineering approach. J Mol Liq 219:194–200. https://doi.org/10.1016/j.molliq.2016.03.018
Drumm FC, Franco DSP, Georgin J et al (2021a) Macro-fungal (Agaricus bisporus) wastes as an adsorbent in the removal of the acid red 97 and crystal violet dyes from ideal colored effluents. Environ Sci Pollut Res 28:405–415. https://doi.org/10.1007/s11356-020-10521-9
Drumm FC, Franco DSP, Grassi P, et al (2021b) Effective adsorptive removal of textile pollutant using coal bottom ash with high surface area obtained by alkaline fusion route. Environ Technol 1–12. https://doi.org/10.1080/09593330.2021.1881828
Elgarahy AM, Elwakeel KZ, Elshoubaky GA, Mohammad SH (2019) Microwave-accelerated sorption of cationic dyes onto green marine algal biomass. Environ Sci Pollut Res 26:22704–22722. https://doi.org/10.1007/s11356-019-05417-2
Elwakeel KZ, Elgarahy AM, Elshoubaky GA, Mohammad SH (2020) Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural). J Environ Health Sci Eng 18:35–50. https://doi.org/10.1007/s40201-019-00435-1
Fadillah G, Yudha SP, Sagadevan S et al (2020) Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications. Open Chem 18:1148–1166
Foroutan R, Peighambardoust SJ, Peighambardoust SH, et al (2021) Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/fe3 o4 magnetic nanocomposite from aqueous solutions: A kinetic, equilibrium and thermodynamic study. Molecules 26. https://doi.org/10.3390/molecules26082241
Franco DSP, Georgin J, Netto MS et al (2021) Conversion of the forest species Inga marginata and Tipuana tipu wastes into biosorbents: Dye biosorption study from isotherm to mass transfer. Environ Technol Innov 22:101521. https://doi.org/10.1016/j.eti.2021.101521
Freundlich H (2017) Über die Adsorption in Lösungen. Z Phys Chem 57U:385–470. https://doi.org/10.1515/zpch-1907-5723
Gapusan RB, Balela MDL (2020) Adsorption of anionic methyl orange dye and lead(II) heavy metal ion by polyaniline-kapok fiber nanocomposite. Mater Chem Phys 243:122682. https://doi.org/10.1016/j.matchemphys.2020.122682
He T, Qiu HJ, Ping CR, Yu L (2021) Adsorption characteristics of methylene blue by a dye-degrading and extracellular polymeric substance -producing strain. J Environ Manag 288:112446. https://doi.org/10.1016/j.jenvman.2021.112446
Hisada M, Tomizawa Y, Kawase Y (2019) Removal kinetics of cationic azo-dye from aqueous solution by poly-γ-glutamic acid biosorbent: Contributions of adsorption and complexation/precipitation to Basic Orange 2 removal. J Environ Chem Eng 7:103157. https://doi.org/10.1016/j.jece.2019.103157
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59:171–177
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Hou M, Li F, Liu X et al (2007) The effect of substituent groups on the reductive degradation of azo dyes by zerovalent iron. J Hazard Mater 145:305–314. https://doi.org/10.1016/j.jhazmat.2006.11.019
Huang H, Liu Z, Yun J et al (2021) Preparation of Laponite hydrogel in different shapes for selective dye adsorption and filtration separation. Appl Clay Sci 201:105936. https://doi.org/10.1016/j.clay.2020.105936
Jabli M, Almalki SG, Agougui H (2020) An insight into methylene blue adsorption characteristics onto functionalized alginate bio-polymer gel beads with λ-carrageenan-calcium phosphate, carboxymethyl cellulose, and celite 545. Int J Biol Macromol 156:1091–1103. https://doi.org/10.1016/j.ijbiomac.2019.11.140
Jagathesan G, Rajiv P (2018) Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity. Biocatal Agric Biotechnol 13:90–94. https://doi.org/10.1016/j.bcab.2017.11.014
Kasperiski FM, Lima EC, do Reis GS et al (2018) Preparation of CTAB-functionalized aqai stalk and its efficient application as adsorbent for the removal of Direct Blue 15 and Direct Red 23 dyes from aqueous media. Chem Eng Commun 205:1520–1536. https://doi.org/10.1080/00986445.2018.1458028
Kaur K, Jindal R (2019) Comparative study on the behaviour of Chitosan-Gelatin based Hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydr Polym 207:398–410. https://doi.org/10.1016/j.carbpol.2018.12.002
Krika F, el Farouk Benlahbib O (2015) Removal of methyl orange from aqueous solution via adsorption on cork as a natural and low-coast adsorbent: equilibrium, kinetic and thermodynamic study of removal process. Desalin Water Treat 53:3711–3723. https://doi.org/10.1080/19443994.2014.995136
Lafi R, Hafiane A (2016) Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste (MCWs). J Taiwan Inst Chem Eng 58:424–433. https://doi.org/10.1016/j.jtice.2015.06.035
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Li B, Wang Q, Guo JZ et al (2018) Sorption of methyl orange from aqueous solution by protonated amine modified hydrochar. Bioresour Technol 268:454–459. https://doi.org/10.1016/j.biortech.2018.08.023
Li Z, Sellaoui L, Gueddida S et al (2020) Adsorption of methylene blue on silica nanoparticles: Modelling analysis of the adsorption mechanism via a double layer model. J Mol Liq 319:114348. https://doi.org/10.1016/j.molliq.2020.114348
Lin J, Su T, Chen J et al (2021) Efficient adsorption removal of anionic dyes by an imidazolium-based mesoporous poly(ionic liquid) including the continuous column adsorption-desorption process. Chemosphere 272:129640. https://doi.org/10.1016/j.chemosphere.2021.129640
Madubuonu N, Aisida SO, Ali A et al (2019) Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study. J Photochem Photobiol B Biol 199:111601. https://doi.org/10.1016/j.jphotobiol.2019.111601
Mansor ES, Ali H, Abdel-Karim A (2020) Efficient and reusable polyethylene oxide/polyaniline composite membrane for dye adsorption and filtration. Colloids Interface Sci Commun 39:100314. https://doi.org/10.1016/j.colcom.2020.100314
Marrakchi F, Hameed BH, Hummadi EH (2020) Mesoporous biohybrid epichlorohydrin crosslinked chitosan/carbon–clay adsorbent for effective cationic and anionic dyes adsorption. Int J Biol Macromol 163:1079–1086. https://doi.org/10.1016/j.ijbiomac.2020.07.032
Melo BC, Paulino FAA, Cardoso VA et al (2018) Cellulose nanowhiskers improve the methylene blue adsorption capacity of chitosan-g-poly(acrylic acid) hydrogel. Carbohydr Polym 181:358–367. https://doi.org/10.1016/j.carbpol.2017.10.079
Mittal H, Al Alili A, Morajkar PP, Alhassan SM (2021) Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J Mol Liq 323:115034. https://doi.org/10.1016/j.molliq.2020.115034
Moradi Z, Madadkar Haghjou M, Zarei M et al (2021) Synergy of production of value-added bioplastic, astaxanthin and phycobilin co-products and Direct Green 6 textile dye remediation in Spirulina platensis. Chemosphere 280:130920. https://doi.org/10.1016/j.chemosphere.2021.130920
Ngabura M, Hussain SA, Ghani WAWA et al (2018) Utilization of renewable durian peels for biosorption of zinc from wastewater. J Environ Chem Eng 6:2528–2539. https://doi.org/10.1016/j.jece.2018.03.052
Noreen S, Khalid U, Ibrahim SM et al (2020) ZnO, MgO and FeO adsorption efficiencies for direct sky Blue dye: Equilibrium, kinetics and thermodynamics studies. J Mater Res Technol 9:5881–5893. https://doi.org/10.1016/j.jmrt.2020.03.115
Oloo CM, Onyari JM, Wanyonyi WC et al (2020) Adsorptive removal of hazardous crystal violet dye form aqueous solution using Rhizophora mucronata stem-barks: Equilibrium and kinetics studies. Environ Chem Ecotoxicol 2:64–72. https://doi.org/10.1016/j.enceco.2020.05.001
Ostrovskii VE (1989) Mechanism of ammonia synthesis over iron catalysts in the equilibrium region. Theor Exp Chem 25:193–201. https://doi.org/10.1007/BF01135010
Pai S, Kini SM, Narasimhan MK et al (2021) Structural characterization and adsorptive ability of green synthesized Fe3O4 nanoparticles to remove Acid blue 113 dye. Surf Interfaces 23:100947. https://doi.org/10.1016/j.surfin.2021.100947
Paiva-Santos AC, Herdade AM, Guerra C et al (2021) Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int J Pharm 597:120311
Pan Z, Lin Y, Sarkar B et al (2019) Green synthesis of iron nanoparticles using red peanut skin extract: Synthesis mechanism, characterization and effect of conditions on chromium removal. J Colloid Interface Sci 558:106–114. https://doi.org/10.1016/j.jcis.2019.09.106
Plachtová P, Medříková Z, Zbořil R et al (2018) Iron and Iron Oxide Nanoparticles Synthesized with Green Tea Extract: Differences in Ecotoxicological Profile and Ability to Degrade Malachite Green. ACS Sustain Chem Eng 6:8679–8687. https://doi.org/10.1021/acssuschemeng.8b00986
Prajapati AK, Mondal MK (2021) Novel green strategy for CuO–ZnO–C nanocomposites fabrication using marigold (Tagetes spp.) flower petals extract with and without CTAB treatment for adsorption of Cr(VI) and Congo red dye. J Environ Manag 290:112615. https://doi.org/10.1016/j.jenvman.2021.112615
Prasad AR, Joseph A (2017) Synthesis, characterization and investigation of methyl orange dye removal from aqueous solutions using waterborne poly vinyl pyrrolidone (PVP) stabilized poly aniline (PANI) core-shell nanoparticles. RSC Adv 7:20960–20968. https://doi.org/10.1039/C7RA01790A
Prasad C, Karlapudi S, Venkateswarlu P et al (2017) Green arbitrated synthesis of Fe3O4 magnetic nanoparticles with nanorod structure from pomegranate leaves and Congo red dye degradation studies for water treatment. J Mol Liq 240:322–328. https://doi.org/10.1016/j.molliq.2017.05.100
Pugazhendhi A, Boovaragamoorthy GM, Ranganathan K et al (2018) New insight into effective biosorption of lead from aqueous solution using Ralstonia solanacearum: Characterization and mechanism studies. J Clean Prod 174:1234–1239. https://doi.org/10.1016/j.jclepro.2017.11.061
Puthukkara PAR, Sunil Jose T, Dinoop lal S (2020) Plant mediated synthesis of zero valent iron nanoparticles and its application in water treatment. J Environ Chem Eng 9:104569. https://doi.org/10.1016/j.jece.2020.104569
Rahim A, Çakir C, Ozturk M et al (2021) Chemical characterization and nutritional value of Spirulina platensis cultivated in natural conditions of Chichaoua region (Morocco). South Afr J Bot 141:235–242. https://doi.org/10.1016/j.sajb.2021.05.006
Rahmani R, Gharanfoli M, Gholamin M et al (2020) Plant-mediated synthesis of superparamagnetic iron oxide nanoparticles (SPIONs) using aloe vera and flaxseed extracts and evaluation of their cellular toxicities. Ceram Int 46:3051–3058. https://doi.org/10.1016/j.ceramint.2019.10.005
Rajumon R, Anand JC, Ealias AM et al (2019) Adsorption of textile dyes with ultrasonic assistance using green reduced graphene oxide: An in-depth investigation on sonochemical factors. J Environ Chem Eng 7:103479. https://doi.org/10.1016/j.jece.2019.103479
Rathika S, Raghavan PS (2021) Adsorption kinetics for the removal of harmful EBT dye by polyvinyl palmitate as effective adsorbents. Mater Today Proc. https://doi.org/10.1016/j.matpr.2021.02.015
Rawat S, Samreen K, Nayak AK et al (2021) Fabrication of iron nanoparticles using Parthenium: A combinatorial eco-innovative approach to eradicate crystal violet dye and phosphate from the aqueous environment. Environ Nanotechnol Monit Manag 15:100426. https://doi.org/10.1016/j.enmm.2021.100426
Raza S, Wen H, Peng Y et al (2021) Fabrication of SiO2 modified biobased hydrolyzed hollow polymer particles and their applications as a removal of methyl orange dye and bisphenol-A. Eur Polym J 144:110199. https://doi.org/10.1016/j.eurpolymj.2020.110199
Rigueto CVT, Piccin JS, Dettmer A et al (2020) Water hyacinth (Eichhornia crassipes) roots, an amazon natural waste, as an alternative biosorbent to uptake a reactive textile dye from aqueous solutions. Ecol Eng 150:105817. https://doi.org/10.1016/j.ecoleng.2020.105817
Saha B, Das S, Saikia J, Das G (2011) Preferential and enhanced adsorption of different dyes on iron oxide nanoparticles: A comparative study. J Phys Chem C 115:8024–8033. https://doi.org/10.1021/jp109258f
Samrot AV, Ali HH, Selvarani AJ et al (2021) Adsorption efficiency of chemically synthesized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) on crystal violet dye. Curr Res Green Sustain Chem 4:100066. https://doi.org/10.1016/j.crgsc.2021.100066
Sellaoui L, Dhaouadi F, Li Z et al (2021) Implementation of a multilayer statistical physics model to interpret the adsorption of food dyes on a chitosan film. J Environ Chem Eng 105516. https://doi.org/10.1016/j.jece.2021.105516
Sharma G, Kumar A, Naushad M et al (2018) Fabrication and characterization of Gum arabic-cl-poly(acrylamide) nanohydrogel for effective adsorption of crystal violet dye. Carbohydr Polym 202:444–453. https://doi.org/10.1016/j.carbpol.2018.09.004
Tran VA, Kadam AN, Lee SW (2020) Adsorption-assisted photocatalytic degradation of methyl orange dye by zeolite-imidazole-framework-derived nanoparticles. J Alloys Compd 835:155414. https://doi.org/10.1016/j.jallcom.2020.155414
Vasantharaj S, Sathiyavimal S, Senthilkumar P et al (2019) Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J Photochem Photobiol B Biol 192:74–82. https://doi.org/10.1016/j.jphotobiol.2018.12.025
Wong JKH, Tan HK, Lau SY et al (2019) Potential and challenges of enzyme incorporated nanotechnology in dye wastewater treatment: A review. J Environ Chem Eng 7:103261
Xiao C, Li H, Zhao Y et al (2020) Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J Environ Manag 275:111262. https://doi.org/10.1016/j.jenvman.2020.111262
Zheng X, Zheng H, Xiong Z et al (2020) Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem Eng J 392:123706. https://doi.org/10.1016/j.cej.2019.123706
Acknowledgements
This work was performed at Faculty of Science, Port-Said University, Port-Said, Egypt. The authors; therefore, acknowledge with thanks the University technical support. Also, the authors would like to thank Prof. Dr. Khaild Zaki Elwakeel, professor of Environmental Chemistry, Environmental Science Department, Faculty of Science, Port Said University, Egypt, for his cooperation and helpful guidance in preparing the revised version.
Author information
Authors and Affiliations
Contributions
Shymaa M. Shalaby: conceptualization, methodology, investigation, and writing original draft.
Fedekar F. Madkour: conceptualization, methodology, and investigation.
Hala Y El-Kassas: conceptualization, methodology, and investigation.
Adel A. Mohamed: conceptualization, methodology, and investigation.
Ahmed M. Elgarahy: conceptualization, investigation, data curation, writing, reviewing, and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study did not use any kind of human participants or human data, which require any kind of approval.
Consent for publication
Our study did not use any kind of individual data such as video and images.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•Green synthesis of iron oxide nanoparticles for efficient sorption of crystal violet (CV) and methyl orange (MO) dyes.
•Maximum sorption capacity close to 256.4 mg g-1 and 270.2 mg g-1 for CV and MO, respectively, fitted by Langmuir equation.
•Fast kinetics (equilibrium~60 min), fitted by pseudo-second order kinetics model.
•Efficient modeling of thermodynamics parameters (endothermic nature).
•Sorbent stability over 5 sorption/desorption cycles; poorly affected by system complexity (good efficiency in tap water and industrial wastewater).
Supplementary materials
ESM 1
(DOCX 492 kb)
Rights and permissions
About this article
Cite this article
Shalaby, .M., Madkour, F.F., El-Kassas, H.Y. et al. Green synthesis of recyclable iron oxide nanoparticles using Spirulina platensis microalgae for adsorptive removal of cationic and anionic dyes. Environ Sci Pollut Res 28, 65549–65572 (2021). https://doi.org/10.1007/s11356-021-15544-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15544-4