Abstract
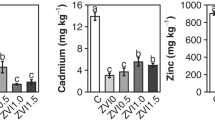
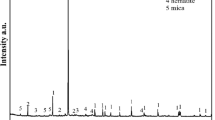
We investigated three common alkaline agents (NaOH, CaO, and Mg(OH)2) for immobilization of four heavy metals (Pb, Zn, Cu, and Cd) in a field-contaminated soil and elucidated the underpinning principles. NaOH caused the highest pH spike in the soil, while CaO and Mg(OH)2 served as a longer-lasting source of OH-. Amending the soil with CaO or Mg(OH)2 at ≥0.1 mol as OH- (kg·soil)−1 for 24 h was able to immobilize all four metals, while NaOH failed. NaOH leached up to 3 times more organic carbon than CaO and Mg(OH)2, resulting in elevated leachability of the metals. Column elution tests showed that amendments by CaO and Mg(OH)2 lowered the leachable Pb2+, Zn2+, Cu2+, and Cd2+ by 52–54%, 71–75%, 69–73%, and 68%, respectively, after 1440 pore volumes of elution. Sequential extraction revealed that the soil amendments converted the exchangeable fraction of the metals to the much less available forms. XRD and FTIR analyses indicated that formation of metal oxide precipitates and complexation with soil organic matter were responsible for the metals immobilization. Taken together the chemical cost, technical effectiveness, and environmental impact, CaO is the most suitable alkaline agent for remediation of soil contaminated with heavy metals.





Similar content being viewed by others
References
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36
Antonakos A, Liarokapis E, Leventouri T (2007) Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 28(19):3043–3054
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manag 105:103–120
Bolan NS, Adriano D, Mani P, Duraisamy A (2003) Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 251(2):187–198
Castaldi P, Santona L, Melis P (2005) Heavy metal immobilization by chemical amendments in a polluted soil and influence on white lupin growth. Chemosphere 60(3):365–371
Cheng S-F, Huang C-Y, Chen K-L, Lin S-C, Lin Y-C (2016) Phytoattenuation of lead-contaminated agricultural land using Miscanthus floridulus-an in situ case study. Desalin Water Treat 57(17):7773–7779
Clay SA, Koskinen WC, Allmaras RR, Dowdy RH (1988) Differences in herbicide adsorption on soil using several soil ph modification techniques. J Environ Sci Health B 23(6):559–573
Coquery M, Welbourn PM (1995) The relationship between metal concentration and organic matter in sediments and metal concentration in the aquatic macrophyte Eriocaulon septangulare. Water Res 29(9):2094–2102
Dong J, Li B, Bao Q (2017) In situ reactive zone with modified Mg(OH)2 for remediation of heavy metal polluted groundwater: immobilization and interaction of Cr(III), Pb(II) and Cd(II). J Contam Hydrol 199:50–57
Du C, Zu Y, Li Y (2007) Effect of liming and pig manure application on fractions of Cd,Pb and Zn in soil and their accumulation in Chinese cabbage. Ecol Environ 16(6):1710–1713
Duarte RMBO, Pio CA, Duarte AC (2005) Spectroscopic study of the water-soluble organic matter isolated from atmospheric aerosols collected under different atmospheric conditions. Anal Chim Acta 530(1):7–14
Feng W, Zhang S, Zhong Q, Wang G, Pan X, Xu X, Zhou W, Li T, Luo L, Zhang Y (2020) Soil washing remediation of heavy metal from contaminated soil with EDTMP and PAA: properties, optimization, and risk assessment. J Hazard Mater 381:120997
Gong Y, Zhao D, Wang Q (2018) An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: technical progress over the last decade. Water Res 147:440–460
He M, Yan P, Yu H, Yang S, Xu J, Liu X (2020) Spatiotemporal modeling of soil heavy metals and early warnings from scenarios-based prediction. Chemosphere 255:126908
Hseu Z-Y, Huang Y-T, Hsi H-C (2014) Effects of remediation train sequence on decontamination of heavy metal-contaminated soil containing mercury. J Air Waste Manage Assoc 64(9):1013–1020
Huang Y-T, Hseu Z-Y, Hsi H-C (2011) Influences of thermal decontamination on mercury removal, soil properties, and repartitioning of coexisting heavy metals. Chemosphere 84(9):1244–1249
Huang Y, Wang M, Li Z, Gong Y, Zeng EY (2019) In situ remediation of mercury-contaminated soil using thiol-functionalized graphene oxide/Fe-Mn composite. J Hazard Mater 373:783–790
Kang D, Son J, Yoo Y, Park S, Huh I-S, Park J (2020) Heavy-metal reduction and solidification in municipal solid waste incineration (MSWI) fly ash using water, NaOH, KOH, and NH4OH in combination with CO2 uptake procedure. Chem Eng J 380:122534
Ke X, Zhang FJ, Zhou Y, Zhang HJ, Guo GL, Tian Y (2020) Removal of Cd, Pb, Zn, Cu in smelter soil by citric acid leaching. Chemosphere 255:126690
Kim W-S, Park G-Y, Kim D-H, Jung H-B, Ko S-H, Baek K (2012) In situ field scale electrokinetic remediation of multi-metals contaminated paddy soil: influence of electrode configuration. Electrochim Acta 86:89–95
Li M, Mohamed I, Raleve D, Chen W, Huang Q (2016) Field evaluation of intensive compost application on Cd fractionation and phytoavailability in a mining-contaminated soil. Environ Geochem Health 38(5):1193–1201
Li H, Wang J, Zhao B, Gao M, Shi W, Zhou H, Xie Z, Zhou B, Lu C, He J (2018) The role of major functional groups: multi-evidence from the binding experiments of heavy metals on natural fulvic acids extracted from lake sediments. Ecotoxicol Environ Saf 162:514–520
Li W, Ni P, Yi Y (2019) Comparison of reactive magnesia, quick lime, and ordinary Portland cement for stabilization/solidification of heavy metal-contaminated soils. Sci Total Environ 671:741–753
Li B, Pu S, Mandal S, Li M (2020a) Viscosity modification enhanced the migration and distribution of colloidal Mg(OH)2 in aquifers contaminated by heavy metals. Environ Int 138:105658
Li F, Yang F, Chen Y, Jin H, Leng Y, Wang J (2020b) Chemical reagent-assisted phytoextraction of heavy metals by Bryophyllum laetivirens from garden soil made of sludge. Chemosphere:126574
Li Z, Gong Y, Zhao D, Dang Z, Lin Z (2021) Simultaneous immobilization of multi-metals in a field contaminated acidic soil using carboxymethyl-cellulose-bridged nano-chlorapatite and calcium oxide. J Hazard Mater 407:124786
Lin J-G, Chen S-Y (1998) The relationship between adsorption of heavy metal and organic matter in river sediments. Environ Int 24(3):345–352
Liu R, Zhao D (2007) Reducing leachability and bioaccessibility of lead in soils using a new class of stabilized iron phosphate nanoparticles. Water Res 41(12):2491–2502
Liu R, Zhao D (2013) Synthesis and characterization of a new class of stabilized apatite nanoparticles and applying the particles to in situ Pb immobilization in a fire-range soil. Chemosphere 91(5):594–601
Liu X, Fu Z, Zhang B, Zhai L, Meng M, Lin J, Zhuang J, Wang GG, Zhang J (2018) Effects of sulfuric, nitric, and mixed acid rain on Chinese fir sapling growth in Southern China. Ecotoxicol Environ Saf 160:154–161
Liu C, Li Z, Berhe AA, Xiao H, Liu L, Wang D, Peng H, Zeng G (2019a) Characterizing dissolved organic matter in eroded sediments from a loess hilly catchment using fluorescence EEM-PARAFAC and UV–Visible absorption: Insights from source identification and carbon cycling. Geoderma 334:37–48
Liu M, Korpelainen H, Dong L, Yi L (2019b) Physiological responses of Elaeocarpus glabripetalus seedlings exposed to simulated acid rain and cadmium. Ecotoxicol Environ Saf 175:118–127
Lombi E, Hamon RE, McGrath SP, McLaughlin MJ (2003) Lability of Cd, Cu, and Zn in polluted soils treated with lime, beringite, and red mud and identification of a non-labile colloidal fraction of metals using isotopic techniques. Environ Sci Technol 37(5):979–984
López-Lara T, López-Cajún C, Alcocer S, Castaño VM (2014) Final distribution of CaO and pH evolution in CaO-treated clays. Water Air Soil Pollut 225(8):1–3
Lu K, Yang X, Gielen G, Bolan N, Ok YS, Niazi NK, Xu S, Yuan G, Chen X, Zhang X, Liu D, Song Z, Liu X, Wang H (2017) Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J Environ Manag 186:285–292
Mantoura RFC, Dickson A, Riley JP (1978) The complexation of metals with humic materials in natural waters. Estuar Coast Mar Sci 6(4):387–408
Mei J, Xie H, Yan XL, Hua LI, Liao XY 2014. Amelioration and reutilization of lead contaminated and acidified farmland. Journal of Agro-Environment Science.
MEP, MLR. 2014. (Ministry of environment protection and ministry of land resources of the people's Republic of China). Nationwide Soil Pollution Survey Report (In Chinese). http://www.mee.gov.cn/gkml/sthjbgw/qt/201404/t20140417_270670.htm
Meunier N, Drogui P, Montane C, Hausler R, Blais J-F, Mercier G (2006) Heavy metals removal from acidic and saline soil leachate using either electrochemical coagulation or chemical precipitation. Journal of Environmental Engineering-Asce 132(5):545–554
Momenian HR, Gholamrezaei S, Salavati-Niasari M, Pedram B, Mozaffar F, Ghanbari D (2013) Sonochemical synthesis and photocatalytic properties of metal hydroxide and carbonate (M: Mg, Ca, Sr or Ba) nanoparticles. J Clust Sci 24(4):1031–1042
Peuravuori J, Pihlaja K (1997) Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal Chim Acta 337(2):133–149
Piccolo A, Spaccini R, De Martino A, Scognamiglio F, di Meo V (2019) Soil washing with solutions of humic substances from manure compost removes heavy metal contaminants as a function of humic molecular composition. Chemosphere 225:150–156
Qayyum MF, MZU R, Ali S, Rizwan M, Naeem A, Maqsood MA, Khalid H, Rinklebe J, Ok YS (2017) Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 174:515–523
Qin, C., Yuan, X., Xiong, T., Tan, Y.Z., Wang, H. 2020. Physicochemical properties, metal availability and bacterial community structure in heavy metal-polluted soil remediated by montmorillonite-based amendments. Chemosphere, 261.
Rosestolato D, Bagatin R, Ferro S (2015) Electrokinetic remediation of soils polluted by heavy metals (mercury in particular). Chem Eng J 264:16–23
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. John Wiley & Sons
Suzuki T, Nakamura A, Niinae M, Nakata H, Fujii H, Tasaka Y (2013) Lead immobilization in artificially contaminated kaolinite using magnesium oxide-based materials: immobilization mechanisms and long-term evaluation. Chem Eng J 232:380–387
Thiers E 2012. Method and system for reduction of scaling in purification of aqueous solutions, Google Patents
Thomas IV, JC, Oladeinde A, Kieran TJ, Finger Jr, JW, Bayona-Vásquez NJ, Cartee JC, Beasley JC, Seaman JC, McArthur JV, Rhodes Jr, OE 2020. Co-occurrence of antibiotic, biocide, and heavy metal resistance genes in bacteria from metal and radionuclide contaminated soils at the Savannah River Site. Microbial biotechnology
Wan Y, Huang Q, Wang Q, Yu Y, Su D, Qiao Y, Li H (2020) Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J Hazard Mater 384:121293
Wang Y, Zhang X, Li R, Lin Y, Liu W, Li R, Zhang Y (2018) Competitive sorption of lead and methylene blue onto black soil and their interaction with dissolved organic matter using two-dimensional correlation analyses. Ecotoxicol Environ Saf 164:484–492
Wang M, Li Y, Zhao D, Zhuang L, Yang G, Gong Y (2020a) Immobilization of mercury by iron sulfide nanoparticles alters mercury speciation and microbial methylation in contaminated groundwater. Chem Eng J 381:122664
Wang Y, Liu Y, Zhan W, Zheng K, Wang J, Zhang C, Chen R (2020b) Stabilization of heavy metal-contaminated soils by biochar: challenges and recommendations. Sci Total Environ 729:139060
Wang Z, Wang H, Wang H, Li Q, Li Y (2020c) Effect of soil washing on heavy metal removal and soil quality: a two-sided coin. Ecotoxicol Environ Saf 203:110981
Xie Z, Du Y, Zeng Y, Li Y, Yan M, Jiao S (2009) Effects of precipitation variation on severe acid rain in southern China. J Geogr Sci 19(4):489–501
Xu X, Hu X, Ding Z, Chen Y (2017) Effects of copyrolysis of sludge with calcium carbonate and calcium hydrogen phosphate on chemical stability of carbon and release of toxic elements in the resultant biochars. Chemosphere 189:76–85
Xu H, Lin H, Jiang H, Guo L (2018) Dynamic molecular size transformation of aquatic colloidal organic matter as a function of pH and cations. Water Res 144:543–552
Yang J, Yang J, Huang J (2017) Role of co-planting and chitosan in phytoextraction of As and heavy metals by Pteris vittata and castor bean - a field case. Ecol Eng 109:35–40
Zhang Z, Lü C, He J, Gao M, Zhao B, Zhou B, Guo J, Zhou H, Liu X, Li Z, Shi W, Jiao Y, Zhao W, Zhang Y (2018) Nature differences of fulvic acid fractions induced by extracted sequence as explanatory factors for binding characteristics of Cu2+. Chemosphere 191:458–466
Zhao XM, Xie H, Kai-Qing WU, Meng-Hao YU, Yang RG, Xiang-Lin LI 2015. Phytoremediation and health risk assessment of acidified and cadmium contaminated farmland. Journal of Agro-Environment Science.
Zhao C, Gao S-J, Zhou L, Li X, Chen X, Wang C-C (2019) Dissolved organic matter in urban forestland soil and its interactions with typical heavy metals: a case of Daxing District, Beijing. Environ Sci Pollut Res 26(3):2960–2973
Zou L, Wang S, Liu L, Hashmi MZ, Tang X, Shi J (2015) Multi-element pollution in soil, ground and surface water from abandoned chromate chemical plants: a case study in Hangzhou, China. Environ Earth Sci 74(4):2861–2870
Acknowledgments
The authors are grateful to Yan Pan, Changdong Ke, Kai Chen, Xiang Peng, Jianle Wang, and Chucheng He for their assistances.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
The study was partially supported by the Guangdong Innovative and Entrepreneurial Research Team Program (No. 2016ZT06N569), Science and Technology Program of Guangzhou (202102010109), the Pearl River Talent Program of Guangdong Province (2017GC010331), the Auburn University IGP program, and the China Scholarship Council (Grant No. CSC201906150048).
Author information
Authors and Affiliations
Contributions
ZLL carried out the experiments, data analysis, data curation, and draft manuscript writing. YG and DZ initiated the concept, advised the research, and finalize the manuscript. ZD and ZL co-supervised the research and acquired and managed partial funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Kitae Baek
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 660 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Gong, Y., Zhao, D. et al. Evaluation of three common alkaline agents for immobilization of multi-metals in a field-contaminated acidic soil. Environ Sci Pollut Res 28, 60765–60777 (2021). https://doi.org/10.1007/s11356-021-14670-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14670-3