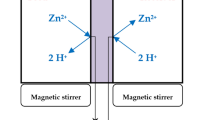
Abstract
This work is focused on the design and preparation of polymer inclusion membranes (PIMs) for potential applications for stannous cation sequestration from water. For this purpose, the membranes have been synthesized employing two polymeric matrices, namely, polyvinylchloride (PVC) and cellulose triacetate (CTA), properly enriched with different plasticizers. The novelty here proposed relies on the modification of the cited PIMs by selected extractants expected to interact with the target cation in the membrane bulk or onto its surface, as well as in the evaluation of their performances in the sequestration of tin(II) in solution through chemometric tools. The composition of both the membrane and the solution for each trial was selected by means of a D-Optimal Experimental Design. The samples such prepared were characterized by means of TG-DTA, DSC, and static contact angles investigations; their mechanical properties were studied in terms of tensile strength and elastic modulus, whereas their morphology was checked by SEM. The sequestering ability of the PIMs toward stannous cation was studied by means of kinetic and isotherm experiments using DP-ASV. The presence of tin in the membranes after the sequestration tests was ascertained by μ-ED-XRF mapping on selected samples.













Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Almeida MIGS, Cattrall RW, Kolev SD (2017) Polymer inclusion membranes (PIMs) in chemical analysis—a review. Anal Chim Acta 987:1–14
Araucz K, Aurich A, Kołodyńska D (2020) Novel multifunctional ion exchangers for metal ions removal in the presence of citric acid. Chemosphere 251:126331
Baby R, Saifullah B, Hussein MZ (2019) Carbon nanomaterials for the treatment of heavy metal-contaminated water and environmental remediation. Nanoscale Res Lett 14:341
Bretti C, Cardiano P, Cigala RM, De Stefano C, Irto A, Lando G, Sammartano S (2018) Exploring various ligand classes for the efficient sequestration of stannous cations in the environment. Sci Total Environ 643:704–714
Cardiano P, Lo Schiavo S, Piraino P (2010) Hydrorepellent properties of organic–inorganic hybrid materials. J Non-Cryst Solids 356:917–926
Cardiano P, Falcone G, Foti C, Giuffrè O, Napoli A (2013) Binding ability of glutathione towards alkyltin(IV) compounds in aqueous solution. J Inorg Biochem 129:84–93
Cardiano P, Foti C, Mineo PG, Galletta M, Risitano F, Lo Schiavo S (2016) Sequestration ability of task specific ionic liquids towards cations of environmental interest. J Mol Liq 223:174–181
Cardiano P, Foti C, Giuffrè O (2017a) Removal of di- and tri-alkyltin(IV) compounds by polyphosphonate ligand: a speciation perspective. J Mol Liq 240:128–137
Cardiano P, Gómez-Laserna O, Mineo PG, Furia E, Triolo C, Patanè S, Lo Schiavo S (2017b) Synthesis, CO2 sorption and capacitive properties of novel protic poly(ionic liquid)s. J Mol Liq 241:222–230
Casella G, Fiore T, Mohamed MMA, Nagy L, Pellerito C, Pellerito L, Sammartano S, Scopelliti M (2007) Equilibria involved in the diorganotin(IV) and triorganotin(IV) phosphomycin interaction in aqueous solution. Appl Organomet Chem 21:455–461
Cataldo S, De Stefano C, Gianguzza A, Pettignano A, Sammartano S (2013) Sequestration of alkyltin(IV) cations by complexation with amino-polycarboxylic chelating agents. J Mol Liq 187:74–82
Cataldo S, Chiodo V, Crea F, Maisano S, Milea D, Pettignano A (2018) Biochar from byproduct to high value added material—a new adsorbent for toxic metal ions removal from aqueous solutions. J Mol Liq 271:481–489
Cerrahoğlu E, Kayan A, Bingöl D (2018) Multivariate optimization for removal of some heavy metals using novel inorganic–organic hybrid and calcined materials. Sep Sci Technol 53:2563–2572
Cigala RM, Crea F, De Stefano C, Lando G, Milea D, Sammartano S (2012) The inorganic speciation of tin(II) in aqueous solution. Geochim Cosmochim Acta 87:1–20
Cole RF (2015) Trends in the analysis and monitoring of organotins in the aquatic environment. Trends Environ Anal 8:1–11
de Carvalho Oliveira R, Santelli RE (2010) Occurrence and chemical speciation analysis of organotin compounds in the environment: a review. Talanta 82:9–24
De Stefano C, Gianguzza A, Marrone F, Piazzese D (1997) Interaction of alkyltin(IV) compounds with ligands of interest in the speciation of natural fluids: complexes of (CH3)2Sn2+ with carboxylates. Appl Organomet Chem 11:683–691
De Stefano C, Foti C, Gianguzza A, Sammartano S (2000) Hydrolysis processes of organotin(IV) compounds in sea water. In: Gianguzza A, Pelizzetti E, Sammartano S (eds) Chemical processes in marine environments. Springer-Verlag, Berlin, pp 213–228
De Stefano C, Gianguzza A, Giuffrè O, Piazzese D, Orecchio S, Sammartano S (2004) Speciation of organotin compounds in NaCl aqueous solution: Interaction of mono-, di- and triorganotin(IV) cations with nucleotides 5' monophosphates. Appl Organomet Chem 18:653–661
Dubalska K, Rutkowska M, Bajger-Nowak G, Konieczka P, Namieśnik J (2013) Organotin compounds: environmental fate and analytics. Crit Rev Anal Chem 43:35–54
Dutta K, De S (2017) Smart responsive materials for water purification: an overview. J Mater Chem A 5:22095–22112
Elias G, Díez S, Fontàs C (2019) System for mercury preconcentration in natural waters based on a polymer inclusion membrane incorporating an ionic liquid. J Hazard Mater 371:316–322
El-Kurd HM, El-Nahhal IM, El-Ashgar NM (2005) Synthesis of new polysiloxane-immobilized ligand system di(amidomethyl)aminetetraacetic acid. Phosphorus Sulfur 180:1657–1671
Foti C, Gianguzza A, Milea D, Millero FJ, Sammartano S (2004) Speciation of trialkyltin(IV) cations in natural fluids. Mar Chem 85:157–167
Gautam RK, Mudhoo A, Lofrano G, Chattopadhyaya MC (2014) Biomass-derived biosorbents for metal ions sequestration: adsorbent modification and activation methods and adsorbent regeneration. J Environ Chem Eng 2:239–259
Gherasim CV, Bourceanu G, Timpu D (2011) Experimental and modeling studies of lead(II) sorption onto a polyvinyl-chloride inclusion membrane. Chem Eng J 172:817–827
Gianguzza A, Giuffrè O, Piazzese D, Sammartano S (2012) Aqueous solution chemistry of alkyltin(IV) compounds for speciation studies in biological fluids and natural waters. Coord Chem Rev 256:222–239
Ho TD, Canestraro AJ, Anderson JL (2011) Ionic liquids in solid-phase microextraction: a review. Anal Chim Acta 695:18–43
Hoffmann F, Cornelius M, Morell J, Fröba M (2006) Silica-based mesoporous organic–inorganic hybrid materials. Angew Chem Int Edit 45:3216–3251
International_Tin_Association 2017: tin industry review International Tin Association, St. Albans (UK)
Jal PK, Patel S, Mishra BK (2004) Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 62:1005–1028
Jon C-S, Meng L-Y, Li D (2019) Recent review on carbon nanomaterials functionalized with ionic liquids in sample pretreatment application. TrAC Trends Anal Chem 120:115641
Kołodyńska D, Budnyak TM, Hubicki Z, Tertykh VA (2017) Sol–gel derived organic–inorganic hybrid ceramic materials for heavy metal removal. In: MAK (ed) Sol–gel based nanoceramic materials: preparation, properties and applications. Springer, Cham
Kuddushi M, Patel NK, Gawali SL, Mata JP, Montes-Campos H, Varela LM, Hassan PA, Malek NI (2020) Thermo-switchable de novo ionogel as metal absorbing and curcumin loaded smart bandage material. J Mol Liq 306:112922
Kuswandi B, Nitti F, Almeida MIGS, Kolev SD (2020) Water monitoring using polymer inclusion membranes: a review. Environ Chem Lett 18:129–150
Laine RM, Babonneau F (1993) Preceramic polymer routes to silicon carbide. Chem Mater 5:260–279
Leardi R (2013) Experimental design. In: Marini F (ed) Chemometrics in food chemistry. Elsevier, Oxford, pp 9–53
Lu F, Astruc D (2018) Nanomaterials for removal of toxic elements from water. Coord Chem Rev 356:147–164
Mahmoud ME, Osman MM, Yakout AA, Abdelfattah AM (2018) Water and soil decontamination of toxic heavy metals using aminosilica-functionalized-ionic liquid nanocomposite. J Mol Liq 266:834–845
Mei M, Huang X, Chen L (2019) Recent development and applications of poly (ionic liquid)s in microextraction techniques. TrAC-Trend. Anal. Chem. 112:123–134
Mishra AK (2016) Smart materials for waste water applications. John Wiley & Sons
Nayak V, Jyothi MS, Balakrishna RG, Padaki M, Deon S (2017) Novel modified poly vinyl chloride blend membranes for removal of heavy metals from mixed ion feed sample. J Hazard Mater 331:289–299
Ngarisan NI, Ngah CWZCW, Ahmad M, Kuswandi B (2014) Optimization of polymer inclusion membranes (PIMs) preparation for immobilization of Chrome Azurol S for optical sensing of aluminum(III). Sensors Actuators B Chem 203:465–470
Nghiem LD, Mornane P, Potter ID, Perera JM, Cattrall RW, Kolev SD (2006) Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J Membr Sci 281:7–41
Okesola BO, Smith DK (2016) Applying low-molecular weight supramolecular gelators in an environmental setting—self-assembled gels as smart materials for pollutant removal. Chem Soc Rev 45:4226–4251
Pellerito L, Nagy L (2002) Organotin(IV)n+ complexes formed with biologically active ligands: equilibrium and structural studies, and some biological aspects. Coord Chem Rev 224:111–150
Peterson J, MacDonell M, Haroun L, Monette F (2007) Tin. In: Hildebrand RD, Taboas A (eds) Human health fact sheet, August 2005. U.S. Department of Energy, pp 54–55
Rosace G, Guido E, Colleoni C, Brucale M, Piperopoulos E, Milone C, Plutino MR (2017) Halochromic resorufin-GPTMS hybrid sol–gel: chemical–physical properties and use as pH sensor fabric coating. Sensors Actuators B Chem 241:85–95
Samiey B, Cheng C-H, Wu J (2014) Organic–inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: a review. Materials 7:673–726
Schapira JP (1997) Le dossier des déschets nucléaires, Société Francaise de Physique
Scheiflinger F, Habibi M, Zeynolabedini E, Plessl C, Wallner G (2019) Radionuclide extraction with different ionic liquids. J Radioanal Nucl Chem 322:1841–1848
Sun J, He B, Yin Y, Li L, Jiang G (2010) Speciation of organotin compounds in environmental samples with semi-permanent coated capillaries by capillary electrophoresis coupled with inductively coupled plasma mass spectrometry. Anal Methods-UK 2:2025–2031
Suresh Kumar D, Susanginee N, Snehaprava D, Kulamani P (2018) Smart 2D-2D Nano-Composite Adsorbents of LDH-carbonaceous materials for the removal of aqueous toxic heavy metal ions: a review. Curr Environ Eng 5:20–34
Tokalıoğlu Ş, Yavuz E, Şahan H, Çolak SG, Ocakoğlu K, Kaçer M, Patat Ş (2016) Ionic liquid coated carbon nanospheres as a new adsorbent for fast solid phase extraction of trace copper and lead from sea water, wastewater, street dust and spice samples. Talanta 159:222–230
Tonle IK, Ngameni E, Njopwouo D, Carteret C, Walcarius A (2003) Functionalization of natural smectite-type clays by grafting with organosilanes: physico-chemical characterization and application to mercury(ii) uptake. Phys Chem Chem Phys 5:4951–4961
Tran HN, You SJ, Chao HP (2016) Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: a comparison study. J Environ Chem Eng 4:2671–2682
Trovato V, Colleoni C, Castellano A, Plutino MR (2018) The key role of 3-glycidoxypropyltrimethoxysilane sol–gel precursor in the development of wearable sensors for health monitoring. J Sol-Gel Sci Technol 87:27–40
Turull M, Elias G, Fontàs C, Díez S (2017) Exploring new DGT samplers containing a polymer inclusion membrane for mercury monitoring. Environ Sci Pollut Res 24:10919–10928
US_Department_of_the_Interior (2020) Tin data sheet—mineral commodity summaries, U.S. Geological Survey
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 290:111197
Vázquez MI, Romero V, Fontàs C, Anticó E, Benavente J (2014) Polymer inclusion membranes (PIMs) with the ionic liquid (IL) Aliquat 336 as extractant: effect of base polymer and IL concentration on their physical–chemical and elastic characteristics. J Membr Sci 455:312–319
Vera R, Gelde L, Anticó E, Martínez de Yuso MV, Benavente J, Fontàs C (2017) Tuning physicochemical, electrochemical and transport characteristics of polymer inclusion membrane by varying the counter-anion of the ionic liquid Aliquat 336. J Membr Sci 529:87–94
Wieszczycka K, Filipowiak K, Wojciechowska I, Aksamitowski P (2020) Novel ionic liquid-modified polymers for highly effective adsorption of heavy metals ions. Sep Purif Technol 236:116313
Wu J, Lee NY (2014) One-step surface modification for irreversible bonding of various plastics with a poly(dimethylsiloxane) elastomer at room temperature. Lab Chip 14:1564–1571
Yadav VB, Gadi R, Kalra S (2019) Clay based nanocomposites for removal of heavy metals from water: a review. J Environ Manag 232:803–817
Yang X, Zhou T, Ren B, Hursthouse A, Zhang Y (2018) Removal of Mn (II) by sodium alginate/graphene oxide composite double-network hydrogel beads from aqueous solutions. Sci Rep 8:10717
Yavir K, Marcinkowski Ł, Marcinkowska R, Namieśnik J, Kloskowski A (2019) Analytical applications and physicochemical properties of ionic liquid-based hybrid materials: a review. Anal Chim Acta 1054:1–16
Zang S, Guo J, Cui A, Li D, Liu D (1996) Measurement of the half life of 126Sn using a radiochemical method. J. Radioanal. Nucl. Ch. 212:93–99
Zawierucha I, Kozlowski C, Malina G (2013) Removal of toxic metal ions from landfill leachate by complementary sorption and transport across polymer inclusion membranes. Waste Manag 33:2129–2136
Funding
This work has been financially supported by the University of Messina (Research&Mobility2017 Project cod. 009041), the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) (PHETRUM Project cod. CTQ2017-82761-P), the European Regional Development Fund (FEDER), and the Italian National Research Council (CNR).
Author information
Authors and Affiliations
Contributions
Gabriele Lando: conceptualization, methodology, writing - reviewing and editing, formal analysis, software, data curation; Olivia Gomez-Laserna: funding acquisition, methodology, writing - reviewing and editing, formal analysis, data curation; Edoardo Proverbio: data curation, writing - reviewing and editing, formal analysis; Amani Khaskhoussi: methodology, investigation, data curation; Daniela Iannazzo: data curation, formal analysis; Maria Rosaria Plutino: methodology, funding acquisition, investigation; Concetta De Stefano: conceptualization, methodology, software, supervision, funding acquisition, resources; Clemente Bretti: conceptualization, validation, investigation, writing - original draft preparation; Paola Cardiano: conceptualization, writing - reviewing and editing, writing - original draft preparation, supervision, methodology, project administration, resources, visualization.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 392 kb)
Rights and permissions
About this article
Cite this article
Lando, G., Gomez-Laserna, O., Proverbio, E. et al. Towards a rational design of materials for the removal of environmentally relevant cations: polymer inclusion membranes (PIMs) and surface-modified PIMs for Sn2+ sequestration in aqueous solution. Environ Sci Pollut Res 28, 51072–51087 (2021). https://doi.org/10.1007/s11356-021-14328-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14328-0