Abstract
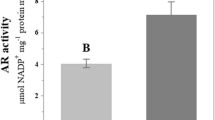
Arsenic (As) is a toxic environmental pollutant. Growing Ricinus communis (castor) on As-contaminated land has the advantage that in addition to revegetation of contaminated land, it can produce bioenergy. To date, As tolerance mechanisms of this plant are not fully understood. In our previous study, we screened tolerant and sensitive genotypes of castor and reported higher total As concentration, enhanced reactive oxygen species (ROS) generation, and oxidative stress in sensitive genotypes of castor GCH 2 and GCH 4 in comparison to tolerant genotypes WM and DCH 177. In the present study, we compared the activity, isoenzyme profile, and gene expression of ROS-scavenging enzymes, proline content, and expression of nicotianamine synthase genes (RcNAS1, RcNAS2, and RcNAS3) in As-tolerant and As-sensitive genotypes of castor. SOD and GPX activity increased significantly in roots of tolerant genotype WM but remained the same or decreased in sensitive genotype GCH 2 and GCH 4 at 200 μM arsenate [As(V)] treatment indicating their important role in As tolerance in castor. CAT activity and proline content increased in sensitive genotypes but remained the same in tolerant genotypes due to As(V) treatment. APX activity showed no significant change in roots and leaves of both tolerant and sensitive genotypes. NAS genes (RcNAS1, RcNAS2, and RcNAS3) encode enzymes that catalyze trimerization of S-adenosylmethionine to form nicotianamine and are critical for metal chelation and heavy metal tolerance. Differential responses of RcNAS1, RcNAS2, and RcNAS3 genes in WM and GCH 2 due to As(V) treatment suggest their role in As(V) tolerance.






Similar content being viewed by others
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi N, Khan M, Amjad M, Hussain M, Natasha (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59. https://doi.org/10.3390/ijerph15010059
Abei H (1981) Measurement of H2O2 in vitro. Methods Enzymol 105:121–124
Adhikari T, Kumar A (2012) Phytoaccumulation and tolerance of Ricinus communis L. to nickel. Int J Phytoremed 14:481–492
Anderegg G, Ripperger G (1989) Correlation between metal complex formation and biological activity of nicotianamine analogues. J Chem Soc Chem Commun 10:647–650
Anjum NA, Hasanuzzaman M, Hossain MA, Thangavel P, Roychoudhury A, Gill SS, Rodrigo MAM, Adam VÄ›, Fujita M, Kizek R, Duarte AC, Pereira E, Ahmad I (2015) Jacks of metal/metalloid chelation trade in plants—an overview. Front Plant Sci 6:192. https://doi.org/10.3389/fpls.2015.00192
Anjum NA, Sharma P, Gill SS, Hasanuzzaman M, Khan EA, Kachhap K, Mohamed AA, Thangavel P, Devi GD, Vasudhevan P, Sofo A, Khan NA, Misra AN, Lukatkin AS, Singh HP, Pereira E, Tuteja N (2016) Catalase and ascorbate peroxidase—representative H2O2-detoxifying heme enzymes in plants. Environ Sci Pollut Res 23:19002–19029
Aung MS, Masuda H, Nozoye T, Kobayashi T, Jeon JS, An G, Nishizawa NK (2019) Nicotianamine synthesis by OsNAS3 is important for mitigating iron excess stress in rice. Front Plant Sci 10:660. https://doi.org/10.3389/fpls.2019.00660
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bauddh K, Singh K, Singh RP (2016) Ricinus communis L. a value added crop for remediation of cadmium contaminated soil. Bull Environ Contam Toxicol 96:265–269
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Begum MC, Islam MS, Islam M, Amin R, Parvez MS, Kabir AH (2016) Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol Biochem 104:266–277
Bharti K, Pandey N, Shankhdhar D, Srivastava PC, Shankhdhar SC (2014) Effect of different zinc levels on activity of superoxide dismutases & acid phosphatases and organic acid exudation on wheat genotypes. Physiol Mol Biol Plants 20:41–48
Bleeker PM, Schat H, Vooijs R, Verkleij JAC, Ernst WHO (2003) Mechanisms of arsenate tolerance in Cytisus striatus. New Phytol 157:33–38
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Chen S, Zhang M, Feng Y, Sahito ZA, Tian S, Yang X (2019) Nicotianamine Synthase Gene 1 from the hyperaccumulator Sedum alfredii Hance is associated with Cd/Zn tolerance and accumulation in plants. Plant Soil 443:413–427
Douchkov D, Gryczka C, Stephan UW et al (2005) Ectopic expression of nicotianamine synthase genes results in improved iron accumulation and increased nickel tolerance in transgenic tobacco. Plant Cell Environ 28:365–374
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta 157:224–232
Gupta M, Sharma P, Sarin NB, Sinha AK (2009) Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere 74:1201–1208
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper- and arsenate-induced oxidative stress in Holcus lanatus L . clones with differential sensitivity. Plant Cell Environ 24:713–722
Hassan Z, Aarts MGM (2011) Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ Exp Bot 72:53–63
Huang S, Van Aken O, Schwarzländer M et al (2016) The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol 171:1551–1559
Jha AB, Sharma P (2019) Regulation of osmolytes syntheses and improvement of abiotic stress tolerance in plants. In: Approaches for Enhancing Abiotic Stress Tolerance in Plants. CRC Press, pp 311–338
Jha AB, Misra AN, Sharma P (2017) Phytoremediation of heavy metal-contaminated soil using bioenergy crops. In: Phytoremediation Potential of Bioenergy Plants. Springer, pp 63–96
Kandpal RP, Rao NA (1985) Alterations in the biosynthesis of proteins and nucleic acids in finger millet (Eleucine coracana) seedlings during water stress and the effect of proline on protein biosynthesis. Plant Sci 40:73–79
Khalid N, Masood A, Noman A, Aqeel M, Qasim M (2019) Study of the responses of two biomonitor plant species (Datura alba & Ricinus communis) to roadside air pollution. Chemosphere 235:832–841
Kim S, Takahashi M, Higuchi K, Tsunoda K, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2005) Increased nicotianamine biosynthesis confers enhanced tolerance of high levels of metals, in particular nickel, to plants. Plant Cell Physiol 46:1809–1818
Kumar JSP, Prasad SR, Banerjee R, Thammineni C (2015) Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann Bot 116:663–668
Kumar K, Gupta D, Mosa KA et al (2019) Arsenic transport, metabolism, and possible mitigation strategies in plants. In: Plant-Metal Interactions. Springer, pp 141–168
Li J, Liu J, Wang G, Cha JY, Li G, Chen S, Li Z, Guo J, Zhang C, Yang Y, Kim WY, Yun DJ, Schumaker KS, Chen Z, Guo Y (2015) A chaperone function of NO CATALASE ACTIVITY1 is required to maintain catalase activity and for multiple stress responses in Arabidopsis. Plant Cell 27:908–925
Luk EE, Culotta VC (2001) Manganese superoxide dismutase in S. cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J Biol Chem 276:47556–47562
Majumder B, Das S, Mukhopadhyay S, Biswas AK (2019) Identification of arsenic-tolerant and arsenic-sensitive rice (Oryza sativa L.) cultivars on the basis of arsenic accumulation assisted stress perception, morpho-biochemical responses, and alteration in genomic template stability. Protoplasma 256:193–211
Mallick S, Sinam G, Sinha S (2011) Study on arsenate tolerant and sensitive cultivars of Zea mays L.: differential detoxification mechanism and effect on nutrients status. Ecotoxicol Environ Saf 74:1316–1324
Maruta T, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S (2010) Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol 51:190–200
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43
Mehes-Smith M, Nkongolo K, Cholewa E (2013) Coping mechanisms of plants to metal contaminated soil. Environ Chang Sustain 54:53–90
Melo EEC, Costa ETS, Guilherme LRG, Faquin V, Nascimento CWA (2009) Accumulation of arsenic and nutrients by castor bean plants grown on an As-enriched nutrient solution. J Hazard Mater 168:479–483
Mishra S, Dubey RS (2006) Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: role of proline as enzyme protectant. J Plant Physiol 163:927–936
Mishra P, Sharma P (2019) Superoxide Dismutases (SODs) and their role in regulating abiotic stress induced oxidative stress in plants. In: Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms, pp 53–88
Mittler R, Zilinskas BA (1993) Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal Biochem 212:540–546
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Oracz K, Karpiński S (2016) Phytohormones signaling pathways and ROS involvement in seed germination. Front Plant Sci 7:864. https://doi.org/10.3389/fpls.2016.00864
Saha J, Majumder B, Mumtaz B, Biswas AK (2017) Arsenic-induced oxidative stress and thiol metabolism in two cultivars of rice and its possible reversal by phosphate. Acta Physiol Plant 39:263. https://doi.org/10.1007/s11738-017-2562-y
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E (2014) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev Environ Contam Toxicol 232:1–44
Sharma SS, Schat H, Vooijs R (1998) In vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry 49:1531–1535
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26
Sharma P, Jha AB, Dubey RS (2014a) Arsenic toxicity and tolerance mechanisms in crop plants. In: Handbook of Plant and Crop Physiology. CRC Press, pp 762–811
Sharma P, Jha AB, Dubey RS, Pessarakli M (2014b) Reactive oxygen species generation, hazards, and defense mechanisms in plants under environmental (abiotic and biotic) stress conditions. In: Handbook of Plant and Crop Physiology. CRC Press, pp 538–577
Sharma P, Srivastava V, Kumar A, Misra AN et al (2018) Mechanisms of metalloid uptake, transport, toxicity, and tolerance in plants. In: Emerging Trends of Plant Physiology for Sustainable Crop Production. Apple Academic Press, pp 167–221
Singh S, Singh R, Bhushan Jha A, Misra AN, Sharma P (2017) Amorphophallus paeoniifolius corm: a potential source of peroxidase for wide applications. Int J Food Prop 20:2658–2664
Singh R, Jha AB, Misra AN, Sharma P (2019a) Differential responses of growth, photosynthesis, oxidative stress, metals accumulation and NRAMP genes in contrasting Ricinus communis genotypes under arsenic stress. Environ Sci Pollut Res 26:31166–31177
Singh R, Jha AB, Misra AN, Sharma P (2019b) Adaption mechanisms in plants under heavy metal stress conditions during phytoremediation. In: Phytomanagement of Polluted Sites. Elsevier, pp 329–360
Singh R, Jha AB, Misra AN, Sharma P (2020a) Differential responses of thiol metabolism and genes involved in arsenic detoxification in tolerant and sensitive genotypes of bioenergy crop Ricinus communis. Protoplasma (online first). https://doi.org/10.1007/s00709-020-01577-y
Singh R, Jha AB, Misra AN, Sharma P (2020b) Entrapment of enzyme in the presence of proline: effective approach to enhance activity and stability of horseradish peroxidase. 3. Biotech 10:1–8
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060
Souri Z, Karimi N, de Oliveira LM (2018) Antioxidant enzymes responses in shoots of arsenic hyperaccumulator, Isatis cappadocica Desv., under interaction of arsenate and phosphate. Environ Technol 39:1316–1327
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, Tandon PK (2007) Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (Lf) Royle. Environ Sci Technol 41:2930–2936
Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inzé D, van Breusegem F (2005) Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol 139:806–821
Wallace G, Fry SC (1999) Action of diverse peroxidases and laccase on six cell wall-related phenolic compounds. Phytochemistry 52:769–773
Wu S, Shen C, Yang Z, Lin B, Yuan J (2016) Tolerance of Ricinus communis L. to Cd and screening of high Cd accumulation varieties for remediation of Cd contaminated soils. Int J Phytoremed 18:1148–1154
Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S (2002) Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J 32:915–925
Zhu D, Scandalios JG (1992) Expression of the maize MnSod (Sod3) gene in MnSOD-deficient yeast rescues the mutant yeast under oxidative stress. Genetics 131:803–809
Acknowledgements
PS is thankful to the Department of Science and Technology-SERB project no. ECR/2016/000888 and University Grant Commission-Start-up grant no. F.4-5(107-FRP)/2014(BSR) for financial support. The Department of Biotechnology (BT/PR9028/INF/22/193/2013) is greatly acknowledged. RS is thankful for UGC-JRF Fellowship during the period of this work. We are very grateful to the reviewers for their valuable comments and suggestions.
Data and materials availability
Not applicable
Funding
The DST-SERB project (ECR/2016/000888), UGC Start-up grant [F. 4-5(107-FRP)/2014 (BSR)], and DBT (BT/PR9028/INF/22/193/2013) supported this work.
Author information
Authors and Affiliations
Contributions
PS—conceived the project; RS—performed all the experiments and statistical analysis; RS and PS—analysed results; RS—wrote the manuscript with the help of PS and ANM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 19.8 kb)
Rights and permissions
About this article
Cite this article
Singh, R., Misra, A.N. & Sharma, P. Effect of arsenate toxicity on antioxidant enzymes and expression of nicotianamine synthase in contrasting genotypes of bioenergy crop Ricinus communis. Environ Sci Pollut Res 28, 31421–31430 (2021). https://doi.org/10.1007/s11356-021-12701-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12701-7