Abstract
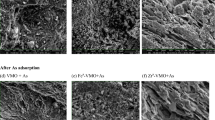
Indigenous hematite iron ore was chemically activated as a function of various hydrogen peroxide concentrations (0.5, 1, 1.5, and 2.0 M), activation time, and iron ore size. Adsorption potential was evaluated at various initial arsenic concentrations, contact time, adsorbent dose, and particle size. Maximum 95% removal efficiency was achieved at 600-μm size of iron ore, activated with 0.5 M concentration of hydrogen peroxide at 24 h of activation time. The experimental data were further evaluated through Langmuir and Freundlich isotherms. The maximum 14.46 mg/g of adsorption capacity was observed through Langmuir isotherm. Moreover, adsorption kinetics was evaluated using pseudo-first-order and pseudo-second-order kinetics, and the intra-particle diffusion model. The kinetics of arsenic adsorption was best described by using the pseudo-first-order kinetics with a kinetic rate of 0.621 min−1. The hematite iron ore before and after arsenic adsorption was characterized by XRD, SEM, and EDX.








Similar content being viewed by others
Data availability
Not applicable.
References
Ahmed S et al (2020) Experimental study and dynamic simulation of melanoidin adsorption from distillery effluent. Environ Sci Pollut Res 27:1–18
Aljeboree AM, Alshirifi AN, Alkaim AF (2017) Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab J Chem 10:S3381–S3393
Asere TG, Stevens CV, Du Laing G (2019) Use of (modified) natural adsorbents for arsenic remediation: a review. Sci Total Environ 676:706–720. https://doi.org/10.1016/j.scitotenv.2019.04.237
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem 2017:11. https://doi.org/10.1155/2017/3039817
Bala P, Torafder P, Azad A, Anwar A (2012) Environment and public health hazard of arsenic and their remedies-a review. Bangladesh Res Pub J 7:69–79
Bazrchi S, Bahram M, Nouri S (2018) Equilibrium and kinetic studies on the removal of acid red-14 from aqueous solutions using PSMA. Iranian Journal of Science and Technology, Transactions A: Science 42:203–208. https://doi.org/10.1007/s40995-018-0489-9
Beyer WF, Fridovich I (1987) Effect of hydrogen peroxide on the iron-containing superoxide dismutase of Escherichia coli. Biochemistry 26:1251–1257. https://doi.org/10.1021/bi00379a008
Bhatti ZA, Qureshi K, Khuhawar MY, Bhutto AW, Ali Z, Solangi INU (2018) Low cost indigenous technologies for removal of arsenic from drinking water: case study of Pakistan. International Journal of Research and Reviews in Applied Sciences 34:01–17. https://www.arpapress.com/Volumes/Vol34Issue3/IJRRAS_34_3_01.pdf
Bhatti ZA, Qureshi K, Maitlo G, Ahmed S (2020) Study of PAN fiber and iron ore adsorbents for arsenic removal. Civil Engineering Journal 6:548–562. https://doi.org/10.28991/cej-2020-03091491
Bibi S, Farooqi A, Yasmin A, Kamran M, Niazi N (2017) Arsenic and fluoride removal by potato peel and rice husk (PPRH) ash in aqueous environments. International Journal of Phytoremediation 19:1029–1036. https://doi.org/10.1080/15226514.2017.1319329
Boddu VM, Abburi K, Talbott JL, Smith ED, Haasch R (2008) Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res 42:633–642
Choong TS, Chuah T, Robiah Y, Koay FG, Azni I (2007) Arsenic toxicity, health hazards and removal techniques from water: an overview. Desalination 217:139–166
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
Edwards M (1994) Chemistry of arsenic removal during coagulation and Fe–Mn oxidation. Journal-American Water Works Association 86:64–78. https://doi.org/10.1002/j.1551-8833.1994.tb06247.x
Engates KE, Shipley HJ (2011) Adsorption of Pb, Cd, Cu, Zn, and Ni to titanium dioxide nanoparticles: effect of particle size, solid concentration, and exhaustion. Environ Sci Pollut Res 18:386–395
Ezzati R (2020) Derivation of pseudo-first-order, pseudo-second-order and modified pseudo-first-order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem Eng J 392:123705
Ghosh S, Debsarkar A, Dutta A (2018) Technology alternatives for decontamination of arsenic-rich groundwater—a critical review Environmental technology & innovation
Goswami A, Raul P, Purkait M (2012) Arsenic adsorption using copper (II) oxide nanoparticles. Chem Eng Res Des 90:1387–1396
Guo H, Stüben D, Berner Z, Kramar U (2008) Adsorption of arsenic species from water using activated siderite-hematite column filters. J Hazard Mater 151:628–635. https://doi.org/10.1016/j.jhazmat.2007.06.035
Guo H, Li Y, Zhao K, Ren Y, Wei C (2011) Removal of arsenite from water by synthetic siderite: behaviors and mechanisms. J Hazard Mater 186:1847–1854
Inyinbor AA, Adekola FA, Olatunji GA (2016) Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resources and Industry 15:14–27. https://doi.org/10.1016/j.wri.2016.06.001
Jadhav SV, Bringas E, Yadav GD, Rathod VK, Ortiz I, Marathe KV (2015) Arsenic and fluoride contaminated groundwaters: a review of current technologies for contaminants removal. J Environ Manag 162:306–325
Jain C, Singh R (2012) Technological options for the removal of arsenic with special reference to South East Asia. J Environ Manag 107:1–18
Jia B, Feng R, Tsau J-S, Barati R (2019) Chapter 15 - Multiphysical flow behavior in shale and permeability measurement by pulse-decay method. In: Cai J, Hu X (eds) Petrophysical characterization and fluids transport in unconventional reservoirs. Elsevier, Amsterdam, pp 301–324. https://doi.org/10.1016/B978-0-12-816698-7.00015-2
Joshi S, Sharma M, Kumari A, Shrestha S, Shrestha B (2019) Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Appl Sci 9:3732
Kleiner DE (2018) 12 - Drugs and Toxins. In: Burt AD, Ferrell LD, Hübscher SG (eds) Macsween’s pathology of the liver, Seventh edn. Elsevier, Amsterdam, pp 673–779. https://doi.org/10.1016/B978-0-7020-6697-9.00012-1
Kwok KC, Koong LF, Al Ansari T, McKay G (2018) Adsorption/desorption of arsenite and arsenate on chitosan and nanochitosan. Environ Sci Pollut Res 25:14734–14742
Liu C-H, Chuang Y-H, Chen T-Y, Tian Y, Li H, Wang M-K, Zhang W (2015) Mechanism of arsenic adsorption on magnetite nanoparticles from water: thermodynamic and spectroscopic studies. Environ Sci Technol 49:7726–7734. https://doi.org/10.1021/acs.est.5b00381
Maji SK, Kao Y-H, Liao P-Y, Lin Y-J, Liu C-W (2013) Implementation of the adsorbent iron-oxide-coated natural rock (IOCNR) on synthetic As(III) and on real arsenic-bearing sample with filter. Appl Surf Sci 284:40–48. https://doi.org/10.1016/j.apsusc.2013.06.154
Min L-L, Zhong L-B, Zheng Y-M, Liu Q, Yuan Z-H, Yang L-M (2016) Functionalized chitosan electrospun nanofiber for effective removal of trace arsenate from water. Sci Rep 6:32480
Mondal P, Hermans N, Kim Tran AT, Zhang Y, Fang Y, Wang X, Van der Bruggen B (2014) Effect of physico-chemical parameters on inorganic arsenic removal from aqueous solution using a forward osmosis membrane. J Environ Chem Eng 2:1309–1316. https://doi.org/10.1016/j.jece.2014.04.015
My Linh NL, Hoang Van D, Duong T, Tinh MX, Quang Khieu D (2019) Adsorption of arsenate from aqueous solution onto modified Vietnamese bentonite. Adv Mater Sci Eng 2019:13. https://doi.org/10.1155/2019/2710926
Nashine A, Tembhurkar A (2016) Equilibrium, kinetic and thermodynamic studies for adsorption of As (III) on coconut (Cocos nucifera L.) fiber. J Environ Chem Eng 4:3267–3273
Nguyen TH et al (2020) Laterite as a low-cost adsorbent in a sustainable decentralized filtration system to remove arsenic from groundwater in Vietnam. Sci Total Environ 699:134267. https://doi.org/10.1016/j.scitotenv.2019.134267
Nwufo BT, Isaac ND, Onche EU (2014) Preparation and characterization of sawdust (cellulose) as an adsorbent for oil pollution remediation. Int J Nat Sci Res 2:97–102
Rahman MA, Hasegawa H, Ueda K, Maki T, Rahman MM (2008) Influence of phosphate and iron ions in selective uptake of arsenic species by water fern (Salvinia natans L.). Chem Eng J 145:179–184
Ray C, Roy PK, Majumder A, Roy MB (2020) Performance analysis of some low-cost and locally available adsorbents that remove excess fluoride ion from raw water to develop a filter for the rural population. Journal of The Institution of Engineers (India): Series E:1–12. https://doi.org/10.1007/s40034-020-00168-z
Ren H-T, Han J, Li T-T, Lin Q, Lin J-H, Lou C-W (2019) Oxalic acid-induced photodissolution of ferrihydrite and the fate of loaded As(V): kinetics and mechanism. Nanomaterials (Basel) 9:1143. https://doi.org/10.3390/nano9081143
Shabani E, Salimi F, Jahangiri A (2019) Removal of arsenic and copper from water solution using magnetic iron/bentonite nanoparticles (Fe3O4/bentonite). Silicon 11:961–971
Shah KH, Ayub M, Fahad M, Bilal M, Amin BAZ, Hussain Z (2019) Natural dolomite as a low-cost adsorbent for efficient removal of As(III) from aqueous solutions. Materials Research Express 6:855–871. https://doi.org/10.1088/2053-1591/ab24f8
Soni R, Shukla DP (2019) Synthesis of fly ash based zeolite-reduced graphene oxide composite and its evaluation as an adsorbent for arsenic removal. Chemosphere 219:504–509. https://doi.org/10.1016/j.chemosphere.2018.11.203
Srivastava D, Vaishya RC (2015) Treatment of arsenic (III) contaminated water by dynamically modified iron-coated sand (DMICS). Desalin Water Treat 53:2565–2577. https://doi.org/10.1080/19443994.2013.867411
Srivastava V, Shekhar M, Gusain D, Gode F, Sharma YC (2017) Application of a heterogeneous adsorbent (HA) for the removal of hexavalent chromium from aqueous solutions: kinetic and equilibrium modeling. Arab J Chem 10:S3073–S3083. https://doi.org/10.1016/j.arabjc.2013.11.049
Swenson H, Stadie NP (2019) Langmuir’s theory of adsorption: a centennial review. Langmuir 35:5409–5426. https://doi.org/10.1021/acs.langmuir.9b00154
Zhang W, Singh P, Paling E, Delides S (2004) Arsenic removal from contaminated water by natural iron ores. Miner Eng 17:517–524
Zheng X, Feng S, Wang X, Shi Z, Mao Y, Zhao Q, Wang S (2019) MSNCs and MgO-MSNCs as drug delivery systems to control the adsorption kinetics and release rate of indometacin. Asian Journal of Pharmaceutical Sciences 14:275–286. https://doi.org/10.1016/j.ajps.2018.08.004
Acknowledgments
The authors are highly thankful to the Mehran University of engineering and technology and Dawood University of engineering and technology for providing experimental facilities for this research study.
Author information
Authors and Affiliations
Contributions
AQM worked on adsorbent preparation and characterization of adsorbent and did batch experimental study.
SA performed the isothermal modeling and used Freundlich and Langmuir modeling approaches.
ZAB worked on the preparation of adsorbent.
GM performed the SEM, EDX, and XRD analyses.
AKH performed batch experimental study and analyzed different operational parameters.
SAM worked on kinetic modeling and observed behavior of intra-particle diffusion.
AM performed pseudo-first-order kinetics and pseudo-second-order kinetics.
ASJ analyzed the effect of H2O2 concentration.
GAK analyzed effect of adsorbent particle size and adsorbent dose.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Memon, A.Q., Ahmed, S., Bhatti, Z.A. et al. Experimental investigations of arsenic adsorption from contaminated water using chemically activated hematite (Fe2O3) iron ore. Environ Sci Pollut Res 28, 12898–12908 (2021). https://doi.org/10.1007/s11356-020-11208-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11208-x