Abstract
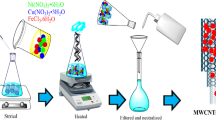
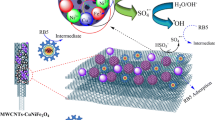
The cobalt ferrite loaded on multi-walled carbon nanotubes (MWCNTs-CoFe2O4) was synthesized and used as a novel catalyst for the degradation of mefenamic acid (MFA) in the presence of peroxymonosulfate (PMS). The results showed that MWCNTs-CoFe2O4 has higher catalytic performance in the activation of PMS and degradation of MFA compared with MWCNTs, Co2+, Fe2+, and CoFe2O4. The highest kinetic constant rate (0.0198 min−1) and MFA degradation (97.63%) were obtained at pH = 7, PMS = 4 mM, catalyst = 500 mg/L, MFA = 10 mg/L, and time = 150 min. MFA degradation accelerated with increasing PMS and catalyst dosage but decreased by initial pH. The influence of different anions and water matrix on the catalytic system was investigated, and the results explained a decrease in the MFA rate in the presence of the interfering substances. Scavenging experiments showed that both sulfate radical anion (SO4•−) and hydroxyl radical (•OH) were effective on MFA degradation, but SO4•− had a greater effect on the degradation of MFA. In addition, the stability and recyclability of MWCNTs-CoFe2O4 were evaluated in the consecutive reaction cycle; the MFA degradation rate reached 89.75% after 4 cycles of reaction. The MFA degradation products were identified by gas chromatography-mass spectrometry (GC-MS) and their degradation pathway was suggested. Finally, a comparison was conducted among the methods used for PMS activation, and the results showed that the cobalt ferrite-based catalyst has high degradation efficiency. However, ultrasound, heat, and ultraviolet (UV) processes can be used to improve the degradation rate of the MWCNTs-CoFe2O4/PMS system at different reaction times.










Similar content being viewed by others
References
Ashrafi S, Mengelizadeh N, Dadban Shahamat Y, Zare MR, Jalil M, Berizi Z, Shooshtarian MR, Parvizimehr A, Zolghadr R (2020) MWCNTs-CoFe2O4 nanoparticles as a reusable novel peroxymonosulfate activator for degradation of reactive black 5. Water Environ Res 92:1–16
Chang E, Liu T-Y, Huang C-P, Liang C-H, Chiang P-C (2012) Degradation of mefenamic acid from aqueous solutions by the ozonation and O3/UV processes. Sep Purif Technol 98:123–129
Chen P, Wang FL, Yao K, Ma JS, Li FH, Lv WY, Liu GG (2016a) Photodegradation of mefenamic acid in aqueous media: kinetics, toxicity and photolysis products. Bull Environ Contam Toxicol 96(2):203–209
Chen J, Zhang L, Huang T, Li W, Wang Y, Wang Z (2016b) Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: radical versus non-radical mechanism. J Hazard Mater 320:571–580
Du Y, Ma W, Liu P, Zou B, Ma J (2016) Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J Hazard Mater 308:58–66
Gao D, Junaid M, Lin F, Zhang S, Xu N (2020) Degradation of sulphachloropyridazine sodium in column reactor packed with CoFe2O4− loaded quartz sand via peroxymonosulfate activation: insights into the amorphous phase, efficiency, and mechanism. Chem Eng J 26:124549
Ghanbari F, Jaafarzadeh N (2017) Graphite-supported CuO catalyst for heterogeneous peroxymonosulfate activation to oxidize direct orange 26: the effect of influential parameters. Res Chem Intermed 43(8):4623–4637
Hong Y, Peng J, Zhao X, Yan Y, Lai B, Yao G (2019) Efficient degradation of atrazine by CoMgAl layered double oxides catalyzed peroxymonosulfate: optimization, degradation pathways and mechanism. Chem Eng J 370:354–363
Huang Y-H, Huang Y-F, C-i H, Chen C-Y (2009) Efficient decolorization of azo dye reactive black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2 + -catalyst. J Hazard Mater 170(2-3):1110–1118
Huang YG, Chen J, Zhang XH, Zan YH, Wu XM, He ZQ, Wang HQ, Li QY (2016) Three-dimensional Co3O4/CNTs/CFP composite as binder-free cathode for rechargeable Li-O2 batteries. Chem Eng J 296:28–34
Huang T, Chen J, Wang Z, Guo X, Crittenden JC (2017) Excellent performance of cobalt-impregnated activated carbon in peroxymonosulfate activation for acid orange 7 oxidation. Environ Sci Pollut Res 24(10):9651–9661
Lassoued A, Dkhil B, Gadri A, Ammar S (2017) Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys 7:3007–3015
Li X, Lu H, Zhang Y, He F (2017) Efficient removal of organic pollutants from aqueous media using newly synthesized polypyrrole/CNTs-CoFe2O4 magnetic nanocomposites. Chem Eng J 316:893–902
Li J, Xu M, Yao G, Lai B (2018) Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: kinetic, degradation intermediates, and toxicity evaluation. Chem Eng J 348:1012–1024
Li Y, Yuan M, Liu H, Sun G (2020a) In situ synthesis of CoFe2O4 nanocrystals decorated in mesoporous carbon nanofibers with enhanced electromagnetic performance. J Alloys Compd 826:154147
Li W, Zhang Y, Zhao P, Zhou P, Liu Y, Cheng X, Wang J, Yang B, Guo H (2020b) Enhanced kinetic performance of peroxymonosulfate/ZVI system with the addition of copper ions: reactivity, mechanism, and degradation pathways. J Hazard Mater 393:122399
Liu J, Zhou J, Ding Z, Zhao Z, Xu X, Fang Z (2017) Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason Sonochem 34:953–959
Long Y, Huang Y, Wu H, Shi X, Xiao L (2019) Peroxymonosulfate activation for pollutants degradation by Fe-N-codoped carbonaceous catalyst: structure-dependent performance and mechanism insight. Chem Eng J 369:542–552
Muhammad S, Saputra E, Sun H, Izidoro JC, Fungaro DA, Ang HM, Tadé MO, Wang S (2012) Coal fly ash supported Co 3 O 4 catalysts for phenol degradation using peroxymonosulfate. RSC Adv 2(13):5645–5650
Othman I, Haija MA, Ismail I, Zain JH, Banat F (2019) Preparation and catalytic performance of CuFe2O4 nanoparticles supported on reduced graphene oxide (CuFe2O4/rGO) for phenol degradation. Mater Chem Phys 238:121931
Pourzamani H, Mengelizadeh N, Hajizadeh Y, Mohammadi H (2018) Electrochemical degradation of diclofenac using three-dimensional electrode reactor with multi-walled carbon nanotubes. Environ Sci Pollut Res 25(25):24746–24763
Pourzamani H, Jafari E, Rozveh M, Mohammadi H, Rostami M, Mengelizadeh N (2019) Degradation of ciprofloxacin in aqueous solution by activating the peroxymonosulfate using graphene based on CoFe2O4. Desalin Water Treat 167:156–169
Qi G, Ren H, Fan H, Liu Y (2019) Preparation of CoFe2O4 nanoparticles based on high-gravity technology and application for the removal of lead. Chem Eng Res Des 147:520–528
Rathod M, Moradeeya PG, Haldar S, Basha S (2018) Nanocellulose/TiO 2 composites: preparation, characterization and application in the photocatalytic degradation of a potential endocrine disruptor, mefenamic acid, in aqueous media. Photochem Photobiol Sci 17(10):1301–1309
Sankoda K, Sugawara Y, Aida T, Yamamoto C, Kobayashi J, Sekiguchi K, Wang Q (2019) Aqueous photochemical degradation of mefenamic acid and triclosan: role of wastewater effluent matrices. Water Sci Technol 79(10):1853–1859
Shahamat YD, Zazouli MA, Zare MR, Mengelizadeh N (2019) Catalytic degradation of diclofenac from aqueous solutions using peroxymonosulfate activated by magnetic MWCNTs-CoFe 3 O 4 nanoparticles. RSC Adv 9(29):16496–16508
Soulet B, Tauxe A, Tarradellas J (2002) Analysis of acidic drugs in Swiss wastewaters. Int J Environ Anal Chem 82(10):659–667
Tan C, Gao N, Fu D, Deng J, Deng L (2017) Efficient degradation of paracetamol with nanoscaled magnetic CoFe2O4 and MnFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Sep Purif Technol 175:47–57
Wang G, Nie X, Ji X, Quan X, Chen S, Wang H, Yu H, Guo X (2019) Enhanced heterogeneous activation of peroxymonosulfate by Co and N codoped porous carbon for degradation of organic pollutants: the synergism between Co and N. Environ Sci Nano 6(2):399–410
Wu J, Cagnetta G, Wang B, Cui Y, Deng S, Wang Y, Huang J, Yu G (2019) Efficient degradation of carbamazepine by organo-montmorillonite supported nCoFe2O4-activated peroxymonosulfate process. Chem Eng J 368:824–836
Xu J, Xin P, Gao Y, Hong B, Jin H, Jin D, Peng X, Li J, Gong J, Ge H (2014) Magnetic properties and methylene blue adsorptive performance of CoFe2O4/activated carbon nanocomposites. Mater Chem Phys 147(3):915–919
Xu L, Chu W, Gan L (2015a) Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer. Chem Eng J 263:435–443
Xu Z, Lu J, Liu Q, Duan L, Xu A, Wang Q, Li Y (2015b) Decolorization of acid orange II dye by peroxymonosulfate activated with magnetic Fe3O4@C/Co nanocomposites. RSC Adv 5(94):76862–76874
Xu L, Wang X, Sun Y, Gong H, Guo M, Zhang X, Meng L, Gan L (2020) Mechanistic study on the combination of ultrasound and peroxymonosulfate for the decomposition of endocrine disrupting compounds. Ultrason Sonochem 60:104749
Yang Z, Li Y, Zhang X, Cui X, He S, Liang H, Ding A (2020) Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate. Chem Eng J 384:123319
Zhang X, Feng M, Qu R, Liu H, Wang L, Wang Z (2016) Catalytic degradation of diethyl phthalate in aqueous solution by persulfate activated with nano-scaled magnetic CuFe2O4/MWCNTs. Chem Eng J 301:1–11
Zhang X, Yao J, Zhao Z, Liu J (2019) Degradation of haloacetonitriles with UV/peroxymonosulfate process: degradation pathway and the role of hydroxyl radicals. Chem Eng J 364:1–10
Zhao F, Zou Y, Lv X, Liang H, Jia Q, Ning W (2015) Synthesis of CoFe2O4–zeolite materials and application to the adsorption of gallium and indium. J Chem Eng Data 60(5):1338–1344
Zhao Y, Nie G, Ma X, Xu P, Zhao X (2019) Peroxymonosulfate catalyzed by rGO assisted CoFe2O4 catalyst for removing Hg0 from flue gas in heterogeneous system. Environ Pollut 249:868–877
Zhou G, Wang Y, Zhou R, Wang C, Jin Y, Qiu J, Hua C, Cao Y (2019) Synthesis of amino-functionalized bentonite/CoFe2O4@ MnO2 magnetic recoverable nanoparticles for aqueous Cd2+ removal. Sci Total Environ 682:505–513
Zhu Y, Chen S, Quan X, Zhang Y (2013) Cobalt implanted TiO 2 nanocatalyst for heterogeneous activation of peroxymonosulfate. RSC Adv 3(2):520–525
Acknowledgments
We are grateful for the financial support provided by the Department of Environmental Health Engineering of Larestan University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadi Amini, M., Mengelizadeh, N. Catalytic degradation of mefenamic acid by peroxymonosulfate activated with MWCNTs-CoFe2O4: influencing factors, degradation pathway, and comparison of activation processes. Environ Sci Pollut Res 27, 45324–45335 (2020). https://doi.org/10.1007/s11356-020-10427-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10427-6