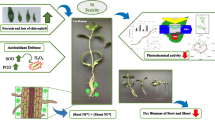
Abstract
The phyto-impact of tungstate is not frequently studied like other heavy metals especially in the sight of continuous accumulation of tungstate in the agriculture soils and water. Thus, the present study was aimed to investigate the supplementation of various tungstate concentrations (0, 1, 5, 10, 50, and 100) to germination water (mg L−1) or clay soil (mg kg−1) on germination and metabolism of broccoli. Lower concentrations (1–10 mg L−1) accelerated germination process and reciprocally were recorded at the highest one (100 mg L−1). The promoter effect of lower concentrations on seedlings growing on tungstate contaminated soil was underpinned from enhancement of pigments, metabolites, enzymatic and non-enzymatic antioxidants, and nitrate reductase. However, the highest concentration-noxious impacts perceived from oxidative damage and membrane integrity deregulation accompanied with no gain from increment of proline, superoxide dismutase, and glutathione-S-transferase. The depletion of phytochelatins and nitric oxide jointed with the enhancement of peroxidases, polyphenol oxidase, and phenylalanine ammonia-lyase at higher concentration reinforced lignin production which restricted plant growth. The results supported the hormetic effects of tungstate (beneficial at low concentrations and noxious at high concentration) on morphological and physiological parameters of broccoli seedlings. The stimulatory effect of tungstate on metabolic activities could serve as important components of antioxidative defense mechanism against tungstate toxicity.







Similar content being viewed by others
Abbreviations
- ASA:
-
Ascorbic acid
- CAT:
-
Catalase
- EL:
-
Electrolyte leakage
- GPX:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- GST:
-
Glutathione-S-transferase
- H2O2 :
-
Hydrogen peroxide
- IPO:
-
Ionic peroxidase
- LOX:
-
Lipoxygenase
- NO:
-
Nitric oxide
- NR:
-
Nitrate reductase
- O2 •− :
-
Superoxide radical
- •OH:
-
Hydroxyl radical
- PAL:
-
Phenylalanine ammonia-lyase
- PCs:
-
Phytochelatins
- PPO:
-
Polyphenol oxidase
- SOD:
-
Superoxide dismutase
- SPO:
-
Soluble peroxidase
- W:
-
Tungsten
References
Achary VMM, Panda BB (2010) Aluminium-induced DNA-damage and adaptive response to genotoxic stress in plant cells are mediated through reactive oxygen intermediates. Mutagenesis 25:201–209
Adamakis IDS, Eleftheriou EP, Rost TL (2008) Effects of sodium tungstate on the ultrastructure and growth of pea (Pisum sativum) and cotton (Gossypium hirsutum) seedlings. Environ Exp Bot 63:416–425. https://doi.org/10.1016/j.envexpbot.2007.12.003
Adamakis IDS, Panteris E, Eleftheriou EP (2011) The fatal effect of tungsten on Pisum sativum L. root cells: indications for endoplasmic reticulum stress-induced programmed cell death. Planta 234(1):21–34
Adamakis IDS, Panteris E, Eleftheriou EP (2012) Tungsten toxicity in plants. Plants 1:82–99. https://doi.org/10.3390/plants1020082
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S00766879(84)05016-3
Babish JG, Stoewsand GS, Furr AK, Parkinson TF, Bache CA, Gutenmann WH, Szolek PCW, Lisk DJ (1979) Elemental and polychlorinated biphenyl content of tissues and intestinal aryl hydrocarbon hydroxylase activity of guinea pigs fed cabbage grown on municipal sewage sludge. J Agric Food Chem 27(2):399–402
Bagy HMK, Hassan EA, Nafady NA, Dawood MFA (2019) Efficacy of arbuscular mycorrhizal fungi and endophytic strain Epicoccum nigrum ASU11 as biocontrol agents against blackleg disease of potato caused by bacterial strain Pectobacterium carotovora subsp. atrosepticum PHY7. Biol Control 134:103–113
Bates LS, Walds RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Brueschweiler B, Waber U, Gupta S (2009) Tungsten, a new vegetable contaminant needs further elaborated evaluation. Toxicol Letters 189:219
Calabrese EJ, Blain RB (2009) Hormesis and plant biology. Environ Pollut 157:42–48
Cataldo DA, Maroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissues by nitration of salicylic acid. Commun Soil Sci Plant Anal 6(1):71–80. https://doi.org/10.1080/00103627509366547
Cervilla LM, Rosales MA, Rubio-Wilhelmi MM, Sánchez-Rodríguez E, Blasco B, Ríos JJ (2009) Involvement of lignification and membrane permeability in the tomato root response to boron toxicity. Plant Sci 176:545–552. https://doi.org/10.1016/j.plantsci.2009.01.008
Chen KM, Gong HJ, Chen GC, Wang SM, Zhang CL (2004) Gradual drought under field conditions influences the glutathione metabolism, redox balance and energy supply in spring wheat. J Plant Growth Regul 23:20–28. https://doi.org/10.1007/s00344-003-0053-4
Chibuike GU, Obiora SC (2014) Bioremediation of hydrocarbon-polluted soils for improved crop performance. Int J Environ Sci 4(5):840–858. https://doi.org/10.6088/ijes.2014040404524
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88(11):707–171
Dago A, Gonzalez I, Arino C, Martinez-Coronado A, Higueras P, Diaz-Cruz JM, Esteban M (2014) Evaluation of mercury stress in plants from the Almadén mining district by analysis of phytochelatins and their Hg complexes. Environ Sci Technol 48(11):6256–6263
Dhindwal AS, Lather BPS, Singh J (1991) Efficacy of seed treatment on germination, seedling emergence and vigor of cotton (Gossypium hirsutum) genotypes. Seed Res 19:59–61
Ding AH, Nathan CF, Stuehr DJ (1998) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol 141:2407–2412
Doster MA, Bostock RM (1988) Quantification of lignin formation in almond bark in response to wounding and infection by Phytophthora species. Phytopathol 78(103–113):473–477
Downs MR, Nadelhoffer K, Melillo JJ, Aber J (1993) Foliar and fine root nitrate reductase activity in seedlings of four forest tree species in relation to nitrogen availability. Trees 7:233–236. https://doi.org/10.1007/BF00202079
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Fales DR (1951) The assimilation and degradation of carbohydrates of yeast cells. J Biol Chem 193:113–118
Flohé L, Günzler WA (1984) Methods in Enzymology. In: Packer L (ed) Assays of glutathione peroxidase. Academic Press, New York, pp 114–121
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17(7):1866–1875. https://doi.org/10.1105/tpc.105.033589
Gall JE, Rajakaruna N (2013) The physiology, functional genomics, and applied ecology of heavy metal-tolerant brassicaceae. In: Lang M (ed) Brassicaceae: characterization, functional genomics and health benefits. Nova Science Publishers, Hauppauge, pp 121–148
Gazizova NV, Petrova FG, Karimova NI (2013) Effect of tungstate on pea root growth and protein tyrosine phosphorylation. Russ J Plant Physiol 60(6):776–784. https://doi.org/10.1134/S1021443713050051
Ghanati F, Morita A, Yokota H (2002) Induction of suberin and increase of lignin content by excess boron in Tabacco cells. Soil Sci Plant Nut 48(3):357–364. https://doi.org/10.1080/00380768.2002.10409212
Ghelfi A, Gaziola SA, Cia MC, Chabregas M, Falco MC, Kuse r-Falcao PR, Azevedo RA (2011) Cloning, expression, molecular modelling and docking analysis of glutathione transferase from Saccharum officinarum. Ann Appl Biol 159(267):280
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Graziano M, Lamattina M (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52:949–960. https://doi.org/10.1111/j.1365-313X.2007.03283.x
Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT (2011) On the origins of nitric oxide. Trends Plant Sci 16(3):160–168. https://doi.org/10.1016/j.tplants.2010.11.007
Habig W, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Haider S, Azmat R (2012) Failure of survival strategies in adaption of heavy metal environment in Lens culinaris and Phaseolus mungo. Pak J Bot 44(6):1959–1964
Hale KL, Tufan HA, Pickering IJ, George GN, Terry N, Pilon M, Pilon-Smits EAH (2002) Anthocyanins facilitate tungsten accumulation in Brassica. Physiol Plant 116(3):351–358. https://doi.org/10.1034/j.1399-3054.2002.1160310.x
Halliwell B, Gutteridge JMC, Arouma OI (1987) The deoxyribose method: a simple test tube assay for the determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165(1):215–219. https://doi.org/10.1016/0003-2697(87)90222-3
Hartikainen H, Xue T, Piironen V (2000) Selenium as an antioxidant and pro-oxidant in ryegrass. Plant Soil 225(1-2):193–200. https://doi.org/10.1023/A:1026512921026
Hasanuzzaman M, Nahar K, Gill SS, Fujita M (2014) Drought stress responses in plants, oxidative stress and antioxidant defense. In: Gill SS, Tuteja N (eds) Climate change and plant abiotic stress tolerance. Wiley, Weinheim, pp 209–249. https://doi.org/10.1002/9783527675265.ch09
Havir EA, Hanson KR (1973) L-phenylalanine ammonia-lyase (maize and potato); evidence that the enzyme is composed of four subunits. Biochem 12(8):1583–1591. https://doi.org/10.1021/bi00732a019
Hu X, Fang J, Cai W, Tang Z (2003) NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chin Sci Bull 48(4):358–363. https://doi.org/10.1007/BF03183230
Jagota SK, Dani HM (1982) Anew colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 127:178–182
Jiang XY, Omarov T, Yesbergenova SZ, Sagi M (2004) The effect of molybdate and tungstate in the growth medium on abscisic acid content and the Mo-hydroxylases activities in barley (Hordeum vulgare L.). Plant Sci 167(2):297–300. https://doi.org/10.1016/j.plantsci.2004.03.025
Kabata-Pendias A, Mukherjee AB (2007) Trace Elements from Soil to Human. Springer, Berlin
Kelly ADR, Lemaire M, Young YK, Eustache JH, Guilbert C, Molina MF, Mann KK (2012) In vivo tungsten exposure alters B cell development and increases DNA damage in murine bone marrow. Toxicol Sci 131(2):434–446. https://doi.org/10.1093/toxsci/kfs324
Kennedy AJ, Johnson DR, Seiter JM, Lindsay JH, Boyd RE, Bednar AJ, Allison PG (2012) Tungsten toxicity, bioaccumulation and compartmentalization into organisms representing two trophic levels. Environ Sci Technol. 46(17):9646–9652. https://doi.org/10.1021/es300606x
Kivcak B, Mert T (2001) Quantitative determination of α-Tocopherol in Arbutus unedo by TLC-densitometry and colorimetry. Fitoterapia 72:656–661. https://doi.org/10.1016/j.fitote.2004.09.021
Koutsospyros A, Braida W, Christodoulatos C, Dermatas D, Strigul N (2006) A review of tungsten: From environmental obscurity to scrutiny. J Hazard Mater 136:1–19
Kühnel D, Scheffler K, Wellner P, Meissner T, Potthoff A, Busch W, Springer A, Schirmer K (2012) Comparative evaluation of particle properties, formation of reactive oxygen species and genotoxic potential of tungsten carbide based nanoparticles in vitro. J Hazard Mater 227–228:418–426
Kumar A, Aery NC (2011) Effect of tungsten on growth, biochemical constituents, molybdenum and tungsten contents in wheat. Plant Soil Environ 57(11):519–525
Kumar A, Aery NC (2012) Effect of tungsten on the growth, dry-matter production, and biochemical constituents of cowpea. Commun Soil Sci Plant Anal 43(7):1098–1107. https://doi.org/10.1080/00103624.2012.656171
Lamhamdi M, Bakrim A, Aarab A, Lafont R, Sayah F (2011) Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. Comptes Rendus Biol 334(2):118–126. https://doi.org/10.1016/j.crvi.2010.12.006
Lassner E, Austria G, Schubert WD (1996) Tungsten, tungsten alloys, and tungsten compounds. In: Elvers B, Hawkins S (eds) Ullmann's Encyclopedia of Industrial Chemistry. VCH, Weinheim, pp A27:229–A27:267
Lavid N, Schwartz A, Yarden O, Tel-Or E (2001) The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of water lily (Nymphaceae). Planta 212:323–331
Lee YP, Takahashi T (1966) An important colorimetric determination of amino acids with the use of ninhydrine. Anal Biochem 14:71–77
Lichtenthaler HK (1987) Chlorophyll and carotenoids pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lombardo MC, Graziano M, Polacco J, Lamattina L (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1:28–33. https://doi.org/10.4161/psb.1.1.2398
Lowry OH, Rosebought NJ, Far AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Bio Chem 193:291–297
L'vov NP, Nosikov AN, Antipov AN (2002) Tungsten-containing enzymes. Biochemistry (Moscow) 67(2):196–200
Madhava Rao KV, Sresty TV (2000) Antioxidative parameters in seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci 157(1):113–128. https://doi.org/10.1016/S0168-9452(00)00273-9
Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM (2018) Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules 23:535. https://doi.org/10.3390/molecules23030535
Minguez-Mosquera MI, Jaren-Galen M, Garrido-Fernandez J (1993) Lipoxygenase activity during pepper ripening and processing of paprika. Phytochem 32(5):1103–1108. https://doi.org/10.1016/S0031-9422(00)95073-8
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:1972–3170
Moerschbacher B, Noll UM, Flott BE, Reisner HJ (1988) Lignin biosynthetic enzymes in stem rust infected, resistant and susceptible near-isogenic Wheat lines Physiology. Mol Plant Pathol 33:33–46
Motsara MR, Roy RN (2008) Guide to laboratory establishment for plant nutrient analysis, FAO fertilizer and plant nutrition bulletin, 19, Food and Agriculture Organization of the United Nations, Rome, Italy. Plant tissue phosphorus determination
Mourato M, Reis R, Martins LL (2012) Characterization of plant antioxidative system in response to abiotic stresses: a focus on heavy metal toxicity. Advances in Selected Plant Physiology Aspects, Giuseppe Montanaro and Bartolomeo Dichio, IntechOpen. https://doi.org/10.5772/34557
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58(2):166–170. https://doi.org/10.1111/j.1399-3054.1983.tb04162.x
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide cross talk: antagonistic effects on cadmium toxicity in mung bean plants through up-regulating the metal detoxification, antioxidant defense, and methylglyoxal detoxification systems. Ecotoxicol Environ Safety 126:245–255. https://doi.org/10.1016/j.ecoenv.2015.12.026
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nakashima J, Awano T, Takeb K, Fujita M, Saiki H (1997) Immunocytochemical localization of phenylalanine ammonia-lyase and cinnamylalcohol dehydrogenase in differentiating tracheary elements derived from Zinnia mesophyll cells. Plant Cell Physiol 38(2):113–123. https://doi.org/10.1093/oxfordjournals.pcp.a029140
Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39:1140–1160
Pandolfini T, Gabbrielli R, Comparini C (1992) Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L. Plant Cell Environ 15(6):719–725
Patel J, Parmar P, Dave B, Subramanian RB (2012) Antioxidative and physiological studies on Colocasia esculentum in response to arsenic stress. Afr J Biotechnol 11(96):16241–16246. https://doi.org/10.5897/AJB11.3263
Patnaik AR, Achary VM, Panda BB (2013) Chromium (VI)-induced hormesis and genotoxicity are mediated through oxidative stress in root cells of Allium cepa L. Plant Growth Regul 71(2):157–170
Pyatt FB, Pyatt AJ (2004) The bioaccumulation of tungsten and copper by organisms inhabiting metalliferous areas in North Queensland, Australia: an evaluation of potential health implications. J Environ Health Res 3:13–18
Ranal MA, De Santana DG, Ferreira WR, Mendes-Rodrigues C (2009) Calculating germination measurements and organizing spreadsheets. Rev Brasil Bot 32(4):849–855. https://doi.org/10.1590/S0100-84042009000400022
Rogers H, Munné-Bosch S (2016) Production and scavenging of reactive oxygen species and redox signaling during leaf and flower senescence: Similar But Different. Plant Physiol 171:1560–1568. https://doi.org/10.1104/pp.16.00163
Sallam A, Alqudah AM, Dawood MF, Baenziger PS, Börner A (2019) Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int J Mol Sci 20(13):31–37
Sánchez-Rodríguez E, Moreno DA, Ferreres F, Rubio-Wilhelmi MM, Ruiz JM (2011) Differential responses of five cherry tomato varieties to water stress: changes on phenolic metabolites and related enzymes. Photochem 72(8):723–729. https://doi.org/10.1016/j.phytochem.2011.02.011
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Rıó LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52(364):2115–2126. https://doi.org/10.1093/jexbot/52.364.2115
Schlegel HG (1956) Die Verwertung Organischer Sauren duch Cholrella in licht. Planta (Berl) 47:510–526. https://doi.org/10.1007/BF01935418
Seiler RL, Stollenwerk KG, Garbarino JR (2005) Factors controlling tungsten concentrations in ground water, Carson Desert, Nevada. Appl Geochem 20(2):423–441. https://doi.org/10.1016/j.apgeochem.2004.09.002
Senesi N, Padovaro G, Brunetti G (1988) Scandium, titanium, tungsten and zirconium content in commercial inorganic fertilizers and their contribution to soil. Environ Techn Lett 9:1011–1020
Seregin IV, Ivanov VB (2001) Physiological aspects of cadmium and lead toxic effects on higher plants. Russ J Plant Physiol 48:523–544. https://doi.org/10.1023/A:1016719901147
Silva EN, Silveira JA, Aragão RM, Vieira CF, Carvalho FE (2019) Photosynthesis impairment and oxidative stress in Jatropha curcas exposed to drought are partially dependent on decreased catalase activity. Acta Physiol Planturm 41(1):4–12. https://doi.org/10.1007/s11738-018-2794-5
Silveira JAG, Araújo SAM, Lima JPMS, Viégas RA (2009) Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in Atriplex nummularia. Environ Exp Bot 66:1–8
Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot 63(1-3):158–167. https://doi.org/10.1016/j.envexpbot.2007.12.005
Strigul NS, Koutsospyros A, Christodoulatos C (2009) Tungsten in the former Soviet Union: Review of environmental regulations and related research. Land Contam Reclam 17:189–215
Thipyapong P, Melkonian J, Wolfe DW, Steffens JC (2004) Suppression of polyphenol oxidases increases stress tolerance in tomato. Plant Sci 167(4):693–703. https://doi.org/10.1016/j.plantsci.2004.04.008
Tian M, Xu X, Hu H, Liu Y, Pan S (2016) Optimization of enzymatic production of sulforaphane in broccoli sprouts and their total antioxidant activity at different growth and storage days. J Food Sci Technol 54:209–218. https://doi.org/10.1007/s13197-016-2452-0
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environm 13:195–206. https://doi.org/10.1111/j.1365-3040.1990.tb01304.x
Vasanthi HR, Mukherjee S, Das DK (2009) Potential health benefits of broccoli—a chemico-biological overview. Mini Rev Med Chem 9:749–759. https://doi.org/10.2174/138955709788452685
Wilson B, Pyatt FB (2009) Persistence and bioaccumulation of tungsten and associated heavy metals under different climatic conditions. Land Contam Reclam 17:93–100
Xiong J, Fu G, Yang Y, Zhu C, Tao L (2012) Tungstate: Is it really a specific nitrate reductase inhibitor in plant nitric oxide research? J Exp Bot 63(1):33–41. https://doi.org/10.1093/jxb/err268
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afri J Bot 76:167–179
Yang H, Wu F, Cheng J (2011) Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem 127:1237–1242. https://doi.org/10.1016/j.foodchem.2011.02.011
Younes NA, Dawood MFA, Wardany AA (2019) Biosafety assessment of graphene nanosheets on leaf ultrastructure, physiological and yield traits of Capsicum annuum L. and Solanum melongena L. Chemosphere 228:318–327. https://doi.org/10.1016/j.chemosphere.2019.04.097
Zornoza P, Vázquez S, Esteban E, Fernández-Pascual M, Carpena R (2002) Cadmium-stress in nodulated white lupin: Strategies to avoid toxicity. Plant Physiol Biochem 40:1003–1009
Zou Y, Lu Y, Wei D (2004) Antioxidant activity of flavonoid-rich extracts of Hypericum perforatum L in vitro. J Agri Food Chem 52:5032–5039. https://doi.org/10.1021/jf049571r
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dawood, M.F.A., Azooz, M.M. Concentration-dependent effects of tungstate on germination, growth, lignification-related enzymes, antioxidants, and reactive oxygen species in broccoli (Brassica oleracea var. italica L.). Environ Sci Pollut Res 26, 36441–36457 (2019). https://doi.org/10.1007/s11356-019-06603-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06603-y