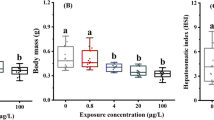
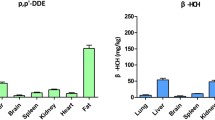
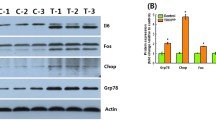
Abstract
As the application and environmental release of tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) are being increased rapidly, serious concerns have been raised regarding its adverse effects on human health. Exposure to TDCIPP has been implicated in hepatotoxicity, but the molecular mechanisms remain unclear. Here, both male and female Sprague Dawley rats were administered TDCIPP with 125, 250, or 500 mg/kg/day for 12 weeks. Then the ultrastructure of liver, biochemical indicators in serum and liver, and hepatic gene expression were analyzed to reveal molecular mechanisms of hepatotoxicity induced by TDCIPP. Continuous TDCIPP exposure decreased body weight, particularly in 500 mg/kg/day TDCIPP-exposed males, and dose dependently increased the ratio of liver to body weight in both genders. The decreased levels of triglyceride, cholesterol, and transaminase in the serum and livers were observed in both genders after TDCIPP exposure, which indicated dysfunction in the hepatic metabolism. Liver histopathology revealed hepatocellular damages in males and females after TDCIPP exposure. The transcriptomic analysis indicated that TDCIPP exposure significantly changed pathways of bile acid metabolism, inflammatory response, oxidative phosphorylation and carcinogenicity in 250 and 500 mg/kg/day TDCIPP-exposed males and 500 mg/kg/day TDCIPP-exposed females, and there was no statistical significance in any other TDCIPP-exposed groups. The transcriptional analysis showed that TDCIPP exposure led to oxidative stress in the livers of rats, thereby increasing the inflammatory response and promoting mechanisms of carcinogenesis in both genders. Finally, TDCIPP led to more severe adverse phenotypic effects in male than female rats.




Similar content being viewed by others
Data availability
The data used to generate the present report are available on request from the corresponding author (13309884463@163.com).
References
Amacher DE (1998) Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul Toxicol Pharmacol 27:119–130
Amacher DE (2002) A toxicologist’s guide to biomarkers of hepatic response. Hum Exp Toxicol 21:253–262
Andresen JA, Grundmann A, Bester K (2004) Organophosphorus flame retardants and plasticisers in surface waters. Sci Total Environ 332:155–166
ATSDR (Agency for Toxic Substances and Disease Registry) (2009) United States Department of Health and Human Services 2009. Draft Toxicological Profile for Phosphate Ester Flame Retardants (September)
Babich MA (2006) CPSC Staff Preliminary Risk Assessment of Flame Retardants (FR) Chemicals in Upholstered Furniture Foam. U.S. Consumer Products Safety Commission, Bethesda
Bacaloni A, Cavaliere C, Foglia P, Nazzari M, Samperi R, Lagana A (2007) Liquid chromatography/tandem mass spectrometry determination of organophosphorus flame retardants and plasticizers in drinking and surface waters. Rapid Commun Mass Spectrom 21:1123–1130
Betts KS (2013) New details on organophosphate flame retardants exposure in men appears stable over time. Environ Health Perspect 121:A168–A168
Brufau G, Groen AK, Kuipers F (2011) Reverse cholesterol transport revisited: contribution of biliary versus intestinal cholesterol excretion. Arterioscler Thromb Vasc Biol 31:1726–1733
Cao SX, Zeng XY, Song H, Li HR, Yu ZQ, Sheng GY, Fu JM (2012) Levels and distributions of organophosphate flame retardants and plasticizers in sediment from Taihu Lake, China. Environ Toxicol Chem 31:1478–1484
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Chen D, Letcher RJ, Chu S (2012) Determination of non-halogenated, chlorinated and brominated organophosphate flame retardants in herring gull eggs based on liquid chromatography-tandem quadrupole mass spectrometry. J Chromatogr A 1220:169–174
Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Crump D, Chiu S, Kennedy SW (2012) Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicol Sci 126:140–148
Dasgupta S, Cheng V, Vliet SMF (2018) Tris(1,3-dichloro-2-propyl) Phosphate exposure during the early-blastula stage alters the normal trajectory of zebrafish embryogenesis. Environ Sci Technol 52:10820–10828
Ding JJ, Xu ZM, Huang W, Feng LM, Yang FX (2016) Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci Total Environ 554-555:211–217
Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, Stapleton HM (2011) Is the PentaBDE replacement, tris (1, 3-dichloro-2-propyl) phosphate (TDCIPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol 256:281–289
Ditzel EJ, Nguyen T, Parker P, Camenisch TD (2016) Effects of arsenite exposure during fetal development on energy metabolism and susceptibility to diet-induced fatty liver disease in male mice. Environ Health Perspect 124:201–209
EFRA (2007) Flame Retardants Frequently Asked Questions. European Flame Retardants Association (EFRA), Brussels
Elgawish RA, Abdelrazek HMA, Ismail SAA, Loutfy NM, Soliman MTA (2019) Hepatoprotective activity of Uncaria tomentosa extract against sub-chronic exposure to fipronil in male rats. Environ Sci Pollut Res 26:199–207
Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, Kennedy SW (2013) In ovo effects of two organophosphate flame retardants-TCPP and TDCIPP-on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci 134:92–104
Farhat A, Buick JK, Williams A, Yauk CL, O'Brien JM, Crump D, Williams KL, Chiu S, Kennedy SW (2014) Tris(1,3-dichloro-2-propyl) phosphate perturbs the expression of genes involved in immune response and lipid and steroid metabolism in chicken embryos. Toxicol Appl Pharmacol 275:104–112
Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C (2007) Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 121:2381–2386
Gebhardt R (2002) Oxidative stress, plant-derived antioxidants and liver fibrosis. Planta Med 68:289–296
Giannini EG, Testa R, Savarino V (2005) Liver enzyme alteration: a guide for clinicians. Can Med Assoc J 172:367–379
Hartmann PC, Burgi D, Giger W (2004) Organophosphate flame retardants and plasticizers in indoor air. Chemosphere 57:781–787
He S, Atkinson C, Qiao F, Cianflone K, Chen X, Tomlinson S (2009) A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Investig 119:2304–2316
Hung CY, Tan CH (2018) TRP channels in nociception and pathological pain 1099:13-27.
Hussain SP, Harris CC (2007) Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 121:2373–2380
Hussain SP, Hofseth LJ, Harris CC (2003) Radical causes of cancer. Nat Rev Cancer 3:276–285
Jaeschke H (2006) Mechanisms of liver injury. II Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol 290:G1083–G1088
Jaeschke H (2011) Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 26:173–179
John YL, Chiang (2009) Bile acids: regulation of synthesis. J Lipid Res 50:1955–1966
John YL, Chiang (2013) Bile acid metabolism and signaling. Compr Physiol 3:1191–1212
Kelany OE, Khaled HE, El-Nahla AM, Abdelrazek HMA, Abdel-Daim MM (2017) Hepato protective and metabolic effects of dietary soy phytoestrogens against hyper caloric diet in cyclic female Wistar rats is mediated through estradiol receptors. Biomed Pharmacol J 10:1061–1069
Kemmlein S, Hahn O, Jann O (2003) Emissions of organophosphate and brominated flame retardants from selected consumer products and building materials. Atmos Environ 37:5485–5493
Klaunig JE (2011) Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol 254:86–99
Kovaleski ES, Goncalves LK, Bortolato G, Marinho JP, Silva LFL, Russo MKB, Agostini F, Funchal C, Dani C (2019) Effects of the ingestion of different kinds of white grape juice (Vitis labrusca) during adolescence on body weight, biochemical parameters and oxidative stress in liver of adult Wistar rats. Food Chem 291:110–116
Leonards P, Steindal EH, Veen IVD (2011) Screening of organophosphor flame retardants 2010. SPFO-Report 1091 TA-2786.
Li TG, Chiang JYL (2014) Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66:948–983
Li RW, Zhou PJ, Guo YY, Lee JS, Zhou BS (2017a) Tris (1,3-dichloro-2-propyl) phosphate induces apoptosis and autophagy in SH-SY5Y cells: involvement of ROS-mediated AMPK/mTOR/ULK1 pathways. Food Chem Toxicol 100:183–196
Li RW, Zhou PJ, Guo YY (2017b) The involvement of autophagy and cytoskeletal regulation in TDCIPP-induced SH-SY5Y cell differentiation. Neurotoxicology 62:14–23
Liu CS, Su GY, Giesy JP, Letcher RJ, Li GY, Agrawal I, Li J, Yu LQ, Wang JH, Gong ZY (2016a) Acute exposure to Tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) causes hepatic inflammation and leads to hepatotoxicity in zebrafish. Sci Rep 6:11
Liu LY, He K, Hites RA, Salamova A (2016b) Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ Sci Technol 50:3065–3073
Marklund A, Andersson B, Haglund P (2005) Organophosphorus flame retardants and plasticizers in air from various indoor environment. J Environ Monit 7:814–819
Martinez-Carballo E, Gonzalez-Barreiro C, Sitka A, Scharf S, Gans O (2007) Determination of selected organophosphate esters in the aquatic environment of Austria. Sci Total Environ 388:290–299
Meeker JD, Cooper EM, Stapleton HM, Hauser R (2013) Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect 121:585–585
Misu H, Takamura T, Matsuzawa N, Shimizu A, Ota T, Sakurai M, Ando H, Arai K, Yamashita T, Honda M, Yamashita T, Kaneko S (2007) Genes involved in oxidative phosphorylation are coordinately upregulated with fasting hyperglycaemia in livers of patients with type 2 diabetes. Diabetologia 50:268–277
Miyake JH, Wang SL, Davis RA (2000) Bile acid induction of cytokine expression by macrophages correlates with repression of hepatic cholesterol 7alpha-hydroxylase. J Biol Chem 275:21805–21808
Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, Di Tullio G, Palasciano G, Moustafa T, Halilbasic E, Trauner M, Moschetta A (2012) Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 142:355–365
NRC (2000) Toxicological Risks of Selected Flame-Retardant Chemicals. Subcommittee on Flame Retardant Chemicals, National Research Council (NRC), National Academy of Sciences, National Academy Press, Washington, DC
OEHHA (2011) Evidence on the Carcinogenicity of Tris(1,3-Dichloro-2-Propyl) Phosphate. Reproductive and Cancer Hazard Assessment Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, Sacramento
Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, Petruzzella V (2012) The oxidative phosphorylation system in mammalian mitochondria. Adv Mitochondrial Med 942:3–37
Papadimitriou E, Loumbourdis NS (2002) Exposure of the frog Rana ridibunda to copper impact on two biomarkers, lipid peroxidation, and glutathione. Bull Environ Contam Toxicol 69:885–891
Pol S, Nalpas B, Vassault A, Bousquet-Lemercier B, Franco D, Lacour B, Berthelot P, Hanoune J, Barouki R (1991) Hepatic activity and mRNA expression of aspartate aminotransferase isoenzymes in alcoholic and nonalcoholic liver disease. Hepatology (Baltimore, Md.) 14:620–625
Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF (2003) The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66:1499–1503
Ren CX, Hu XG, Zhou QX (2018) Graphene oxide quantum dots reduce oxidative stress and inhibit neurotoxicity in vitro and in vivo through catalase-like activity and metabolic regulation. Adv Sci 5:13
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer how are they linked? Free Radic Biol Med 49:1603–1616
Sandoval M, Okuhama NN, Zhang XJ, Condezo LA, Lao J, Angeles’ FM, Musah RA, Bobrowski P, Miller MJ (2002) Antiinflammatory and antioxidant activities of cat’s claw (Uncaria tomentosa and Uncaria guianensis) are independent of their alkaloid content. Phytomedicine 9:325–337
Stackelberg PE, Furlong ET, Meyer MT, Zaugg SD, Henderson AK, Reissman DB (2004) Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci Total Environ 329:99–113
Sundkvist AM, Olofsson U, Haglund P (2010) Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit 12:943–951
Teoh N, Field J, Sutton J, Farrell G (2004) Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-alpha gene knockout mice. Hepatology 39:412–421
van den Eede N, Dirtu AC, Neels H, Covaci A (2011) Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int 37:454–461
van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity, and analysis. Chemosphere 88:1119–1153
Vasconcelos SML, Goulart MOF, de Moura JBF, Manfredini V, Benfato M da S, Kubota LT (2007) Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: Principais métodos analíticos para sua determinação. Quím Nova 30:1323–1338
Vuković A, Vuković R, Vitić Z, Vuković K, Štolfa L, Blažetić S, Balog M, Heffer M, Has-Schön E (2016) Sex-specific oxidative-antioxidative status in the liver of the chronically and acutely stressed rats. Bridges in life Sciences, 11th Annual Scientific conference - RECOOP HST ASSOCIATION
Wang XW, Liu JF, Yin YG (2011) Development of an ultra-high-performance liquid chromatography-tandem mass spectrometry method for high throughput determination of organophosphorus flame retardants in environmental water. J Chromatogr A 1218:6705–6711
WHO (World Health Organization) (1998) EHC 209: Flame Retardants: Tris-(Chloropropyl) Phosphate and Tris-(2-Chloroethyl) phosphate. Switzerland, Geneva
Zhang YK, Li M, Li SY, Wang QW, Zhu GN, Su GY, Letcher RJ, Liu CS (2018) Exposure to tris(1,3-dichloro-2-propyl) phosphate for two generations decreases fecundity of zebrafish at environmentally relevant concentrations. Aquat Toxicol 200:178–187
Zhao F, Wang J, Fang YJ, Ding J, Yang HL, Li L, Xi ZG, Qiao HX (2016) Effects of tris(1,3-dichloro-2-propyl) phosphate on pathomorphology and gene/protein expression related to thyroid disruption in rats. Toxicol Res 5:921–930
Zhou QX, Kong FX, Zhu L (eds) (2004) Ecotoxicology. Science Press, Beijing
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
The authors are grateful for the financial support by the National Natural Science Foundation of China (Grant Nos. 31670508 and 21677080), the Ministry of Education, People’s Republic of China as an innovative team project (Grant No. IRT_17R58), and the 111 program (T2017002), special funds for basic scientific research services of central colleges and universities. We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 7096 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Hu, X. & Li, X. Sub-chronic exposure to Tris(1,3-dichloro-2-propyl) phosphate induces sex-dependent hepatotoxicity in rats. Environ Sci Pollut Res 26, 33351–33362 (2019). https://doi.org/10.1007/s11356-019-06383-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06383-5