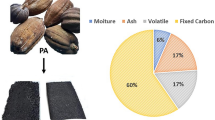
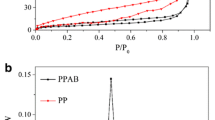
Abstract
A novel biosorbent Phanera vahlii fruit biomass (PVF) and its biochar and chemically modified forms were studied for the elimination of Cr(VI) from synthetic solutions. Biosorbents were characterized through BET, FTIR, FESEM, EDX, and TGA technique. The parameters influencing biosorption were optimized and found as pH 2.0, temperature 303 K, initial metal concentration 500 mg/L, and biosorbent dosage 0.5 g/L. The ideal contact time was 180 min for all biosorbents. Freundlich isotherm was found to have good correlation with investigational data, which indicated that biosorption takes place in multiple layer style. Langmuir adsorption isotherm yielded the highest biosorption capacity (Qo) to be 159.1, 225.1, 244.1, and 278.5 mg/g for Phanera vahlii fruit biomass, Phanera vahlii biochar, Phanera vahlii phosphoric acid activated carbon, and Phanera vahlii zinc chloride activated carbon, respectively. Experimental data had good correlation with pseudo-second-order kinetic model fitted. Thermodynamic studies indicated the biosorption process to be spontaneous, stable, and endothermic. Thus, it was concluded that Phanera vahlii fruit biomass and the derived activated carbons are promising biosorbents for adsorption of chromium from aqueous solutions.

Graphical abstract









Similar content being viewed by others
Abbreviations
- q t :
-
Biosorption capacity (mg/g)
- q e :
-
Biosorption capacity at equilibrium (mg/g)
- C o :
-
Initial metal concentration (mg/L)
- C e :
-
Metal concentration at equilibrium (mg/L)
- C t :
-
Metal concentration at t time (mg/L)
- V:
-
Volume of the metal solution (L)
- m:
-
Weight of the biosorbent (g)
- Q o :
-
Biosorption capacity from Langmuir model (mg/g)
- K L :
-
Langmuir isotherm constant (L/mg)
- R L :
-
Separation factor (dimensionless)
- K F :
-
Freundlich isotherm constant (mg/g) (L/mg)1/n
- n F :
-
Freundlich exponent (dimensionless)
- Q m :
-
Maximum biosorption capacity from Dubinin–Radushkevich model (mg/g)
- K :
-
Constant related to the mean free energy of biosorption (mol2/kJ2)
- ɛ :
-
Polanyi potential of Dubinin–Radushkevich model (kJ/mol)
- R :
-
Universal gas constant (8.314 J/mol/K)
- T :
-
Temperature (K)
- E :
-
Apparent adsorption energy (kJ/mol)
- k 1 :
-
Pseudo-first-order constant (min−1)
- k 2 :
-
Pseudo-second-order constant (g/mg/min)
- k id :
-
Intraparticle diffusion rate constant (mg/g/min1/2)
- C :
-
Intercept of intraparticle diffusion model
- ΔG°:
-
Free energy change (kJ/mol)
- ΔH°:
-
Enthalpy change (kJ/mol)
- ΔS°:
-
Entropy change (kJ/mol/K)
- K c :
-
Distribution coefficient
References
Ajmani A, Narayanan S, Patra C, Narayanasamy S (2018) Studies on the remediation of chromium (VI) from simulated wastewater using novel biomass of Pinus kesiya cone. Desalin Water Treat 114:192–204. https://doi.org/10.5004/dwt.2018.22321
Banerjee M, Basu RK, Das SK (2018) Cr(VI) adsorption by a green adsorbent walnut shell: adsorption studies, regeneration studies, scale-up design and economic feasibility. Process Saf Environ Prot 116:693–702. https://doi.org/10.1016/j.psep.2018.03.037
Barnie S, Zhang J, Wang H, Yin H, Chen H (2018) The influence of pH, co-existing ions, ionic strength, and temperature on the adsorption and reduction of hexavalent chromium by undissolved humic acid. Chemosphere 212:209–218. https://doi.org/10.1016/j.chemosphere.2018.08.067
Daneshvar M, Hosseini MR (2018) Kinetics, isotherm, and optimization of the hexavalent chromium removal from aqueous solution by a magnetic nanobiosorbent. Environ Sci Pollut Res 25:28654–28666. https://doi.org/10.1007/s11356-018-2878-1
Dehghani MH, Taher MM, Bajpai AK, Heibati B, Tyagi I, Asif M, Agarwal S, Gupta VK (2015) Removal of noxious Cr (VI) ions using single-walled carbon nanotubes and multi-walled carbon nanotubes. Chem Eng J 279:344–352. https://doi.org/10.1016/j.cej.2015.04.151
Domingues RR, Trugilho PF, Silva CA, de Melo ICNA, Melo LCA, Magriotis ZM, Sánchez-Monedero MA (2017) Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS One 12:e0176884. https://doi.org/10.1371/journal.pone.0176884
Fan H-T, Shi L-Q, Shen H, Chen X, Xie K-P (2016a) Equilibrium, isotherm, kinetic and thermodynamic studies for removal of tetracycline antibiotics by adsorption onto hazelnut shell derived activated carbons from aqueous media. RSC Adv 6:109983–109991. https://doi.org/10.1039/C6RA23346E
Fan H-T, Sun W, Jiang B, Wang Q-J, Li D-W, Huang C-C, Wang K-J, Zhang Z-G, Li W-X (2016b) Adsorption of antimony(III) from aqueous solution by mercapto-functionalized silica-supported organic–inorganic hybrid sorbent: mechanism insights. Chem Eng J 286:128–138. https://doi.org/10.1016/j.cej.2015.10.048
Fan H-T, Zhao C-Y, Liu S, Shen H (2017) Adsorption characteristics of chlorophenols from aqueous solution onto graphene. J Chem Eng Data 62:1099–1105. https://doi.org/10.1021/acs.jced.6b00918
Feng Z, Chen N, Feng C, Gao Y (2018) Mechanisms of Cr(VI) removal by FeCl3-modified lotus stem-based biochar (FeCl3@LS-BC) using mass-balance and functional group expressions. Colloids Surf Physicochem Eng Asp 551:17–24. https://doi.org/10.1016/j.colsurfa.2018.04.054
Freundlich H (1907) Über die Adsorption in Lösungen. Z Für Phys Chem 57U:385–470. https://doi.org/10.1515/zpch-1907-5723
Guidelines for Drinking-water Quality, 4th ed, 2011. World Health Organization (WHO), Geneva
Gupta VK, Pathania D, Agarwal S, Sharma S (2013a) Removal of Cr(VI) onto Ficus carica biosorbent from water. Environ Sci Pollut Res Int 20:2632–2644. https://doi.org/10.1007/s11356-012-1176-6
Gupta VK, Pathania D, Sharma S, Singh P (2013b) Preparation of bio-based porous carbon by microwave assisted phosphoric acid activation and its use for adsorption of Cr(VI). J Colloid Interface Sci 401:125–132. https://doi.org/10.1016/j.jcis.2013.03.020
Han R, Zhang J, Zou W, Shi J, Liu H (2005) Equilibrium biosorption isotherm for lead ion on chaff. J Hazard Mater 125:266–271. https://doi.org/10.1016/j.jhazmat.2005.05.031
Han R, Li H, Li Y, Zhang J, Xiao H, Shi J (2006) Biosorption of copper and lead ions by waste beer yeast. J Hazard Mater 137:1569–1576. https://doi.org/10.1016/j.jhazmat.2006.04.045
Han R, Zhang L, Song C, Zhang M, Zhu H, Zhang LJ (2010) Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr Polym 79:1140–1149. https://doi.org/10.1016/j.carbpol.2009.10.054
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Jiao Y, Han D, Lu Y, Rong Y, Fang L, Liu Y, Han R (2017) Characterization of pine-sawdust pyrolytic char activated by phosphoric acid through microwave irradiation and adsorption property toward CDNB in batch mode. Desalin Water Treat 77:247–255. https://doi.org/10.5004/dwt.2017.20780
Kayalvizhi K, Vijayaraghavan K, Velan M (2015) Biosorption of Cr(VI) using a novel microalga Rhizoclonium hookeri: equilibrium, kinetics and thermodynamic studies. Desalin Water Treat 56:194–203. https://doi.org/10.1080/19443994.2014.932711
Khalid R, Aslam Z, Abbas A, Ahmad W, Ramzan N, Shawabkeh R (2018) Adsorptive potential of Acacia nilotica based adsorbent for chromium(VI) from an aqueous phase. Chin J Chem Eng 26:614–622. https://doi.org/10.1016/j.cjche.2017.08.017
Khosravi R, Fazlzadehdavil M, Barikbin B, Taghizadeh AA (2014) Removal of hexavalent chromium from aqueous solution by granular and powdered Peganum harmala. Appl Surf Sci 292:670–677. https://doi.org/10.1016/j.apsusc.2013.12.031
Kumar A, Jena HM (2017) Adsorption of Cr(VI) from aqueous solution by prepared high surface area activated carbon from fox nutshell by chemical activation with H3PO4. J Environ Chem Eng 5:2032–2041. https://doi.org/10.1016/j.jece.2017.03.035
Kuppusamy S, Thavamani P, Megharaj M, Venkateswarlu K, Lee YB, Naidu R (2016) Potential of Melaleuca diosmifolia leaf as a low-cost adsorbent for hexavalent chromium removal from contaminated water bodies. Process Saf Environ Prot 100:173–182. https://doi.org/10.1016/j.psep.2016.01.009
Labied R, Benturki O, Eddine Hamitouche AY, Donnot A (2018) Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): kinetic, equilibrium, and thermodynamic study. Adsorpt Sci Technol 36:1066–1099. https://doi.org/10.1177/0263617417750739
Lagergren SY (1898) Zur Theorie der sogenannten Adsorption gelöster Stoffe. Bih Till K Sven Vetenskapsakademiens Handl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Li S, Qi F, Xiao M, Fan H, Shen Y, Du K, Zhang Z, Li W (2017) In situ synthesis of layered double hydroxides on γ-Al2O3 and its application in chromium(VI) removal. Water Sci Technol 75:1466–1473. https://doi.org/10.2166/wst.2017.012
Lin C, Luo W, Luo T, Zhou Q, Li H, Jing L (2018) A study on adsorption of Cr (VI) by modified rice straw: characteristics, performances and mechanism. J Clean Prod 196:626–634. https://doi.org/10.1016/j.jclepro.2018.05.279
Liu Y (2009) Is the free energy change of adsorption correctly calculated? J Chem Eng Data 54:1981–1985. https://doi.org/10.1021/je800661q
Liu Y, Liu Y-J (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61:229–242. https://doi.org/10.1016/j.seppur.2007.10.002
Liu Y, Xu H (2007) Equilibrium, thermodynamics and mechanisms of Ni2+ biosorption by aerobic granules. Biochem Eng J 35:174–182. https://doi.org/10.1016/j.bej.2007.01.020
Milonjic S (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serbian Chem Soc 72:1363–1367. https://doi.org/10.2298/JSC0712363M
Morris JC, Weber WJ (1964) Removal of biologically-resistant pollutants from waste waters by adsorption. In: Southgate BA (ed) Advances in water pollution research. Pergamon, pp 231–266. https://doi.org/10.1016/B978-1-4832-8391-3.50032-4
Moussavi G, Barikbin B (2010) Biosorption of chromium(VI) from industrial wastewater onto pistachio hull waste biomass. Chem Eng J 162:893–900. https://doi.org/10.1016/j.cej.2010.06.032
Nakkeeran E, Selvaraju N (2017) Biosorption of chromium(VI) in aqueous solutions by chemically modified Strychnine tree fruit shell. Int J Phytoremediation 19:1065–1076. https://doi.org/10.1080/15226514.2017.1328386
Niazi L, Lashanizadegan A, Sharififard H (2018) Chestnut oak shells activated carbon: preparation, characterization and application for Cr (VI) removal from dilute aqueous solutions. J Clean Prod 185:554–561. https://doi.org/10.1016/j.jclepro.2018.03.026
Norouzi S, Heidari M, Alipour V, Rahmanian O, Fazlzadeh M, Mohammadi-moghadam F, Nourmoradi H, Goudarzi B, Dindarloo K (2018) Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from date press cake; an agro-industrial waste. Bioresour Technol 258:48–56. https://doi.org/10.1016/j.biortech.2018.02.106
Omidvar Borna M, Pirsaheb M, Vosoughi Niri M, Khosravi Mashizie R, Kakavandi B, Zare MR, Asadi A (2016) Batch and column studies for the adsorption of chromium(VI) on low-cost Hibiscus cannabinus kenaf, a green adsorbent. J Taiwan Inst Chem Eng 68:80–89. https://doi.org/10.1016/j.jtice.2016.09.022
Prabhakaran SK, Vijayaraghavan K, Balasubramanian R (2009) Removal of Cr(VI) ions by spent tea and coffee dusts: reduction to Cr(III) and biosorption. Ind Eng Chem Res 48:2113–2117. https://doi.org/10.1021/ie801380h
Rangabhashiyam S, Selvaraju N (2015) Adsorptive remediation of hexavalent chromium from synthetic wastewater by a natural and ZnCl2 activated Sterculia guttata shell. J Mol Liq 207:39–49. https://doi.org/10.1016/j.molliq.2015.03.018
Rodrigues DAS, Moura JM, Dotto GL, Cadaval TRS, Pinto LAA (2018) Preparation, characterization and dye adsorption/reuse of chitosan-vanadate films. J Polym Environ 26:2917–2924. https://doi.org/10.1007/s10924-017-1171-6
Saranya N, Ajmani A, Sivasubramanian V, Selvaraju N (2018) Hexavalent chromium removal from simulated and real effluents using Artocarpus heterophyllus peel biosorbent - batch and continuous studies. J Mol Liq 265:779–790. https://doi.org/10.1016/j.molliq.2018.06.094
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198557040603
Specimen View [WWW Document], n.d. URL http://chandigarhforestflora.in/specimen/view/5 (accessed 9.10.18)
Tran HN, You S-J, Chao H-P (2016) Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: a comparison study. J Environ Chem Eng 4:2671–2682. https://doi.org/10.1016/j.jece.2016.05.009
Vakili M, Deng S, Li T, Wang W, Wang W, Yu G (2018) Novel crosslinked chitosan for enhanced adsorption of hexavalent chromium in acidic solution. Chem Eng J 347:782–790. https://doi.org/10.1016/j.cej.2018.04.181
Yacou C, Altenor S, Carene B, Gaspard S (2018) Chemical structure investigation of tropical Turbinaria turbinata seaweeds and its derived carbon sorbents applied for the removal of hexavalent chromium in water. Algal Res 34:25–36. https://doi.org/10.1016/j.algal.2018.06.014
Yang J, Yu M, Chen W (2015) Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: kinetics, equilibrium and thermodynamics. J Ind Eng Chem 21:414–422. https://doi.org/10.1016/j.jiec.2014.02.054
Yang H-D, Zhao Y-P, Li S-F, Fan X, Wei X-Y, Zong Z-M (2016) Removal of hexavalent chromium from aqueous solution by calcined Zn/Al-LDHs. Water Sci Technol 74:229–235. https://doi.org/10.2166/wst.2016.204
You N, Li J-Y, Fan H-T, Shen H (2019a) In-situ sampling of nitrophenols in industrial wastewaters using diffusive gradients in thin films based on lignocellulose-derived activated carbons. J Adv Res 15:77–86. https://doi.org/10.1016/j.jare.2018.09.005
You N, Song Y-X, Wang H-R, Kang L-X, Fan H-T, Yao H (2019b) Sol–gel derived benzo-crown ether-functionalized silica gel for selective adsorption of Ca2+ ions. J Chem Eng Data 64:1378–1384. https://doi.org/10.1021/acs.jced.8b00955
You N, Wang X-F, Li J-Y, Fan H-T, Shen H, Zhang Q (2019c) Synergistic removal of arsanilic acid using adsorption and magnetic separation technique based on Fe3O4@ graphene nanocomposite. J Ind Eng Chem 70:346–354. https://doi.org/10.1016/j.jiec.2018.10.035
Zhou L, Zhang G, Wang M, Wang D, Cai D, Wu Z (2018) Efficient removal of hexavalent chromium from water and soil using magnetic ceramsite coated by functionalized nano carbon spheres. Chem Eng J 334:400–409. https://doi.org/10.1016/j.cej.2017.10.065
Acknowledgments
The Central Instrumentation Facility, Indian Institute of Technology Guwahati is highly appreciated for the technical assistance.
Funding
This study received monetary assistance from the Indian Institute of Technology Guwahati, India (Grant No. BSBESUGIITG01213xSEN001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Novel biosorbent Phanera vahlii fruit biomass and derived activated carbons were utilized for Cr(VI) removal.
• Characterization was done using FTIR, FESEM, and EDX.
• BET studies revealed large surface area for activated carbons.
• Phanera vahlii Zinc chloride activated carbon was found to have high adsorption capacity for Cr(VI) of 297.61 mg/g.
Rights and permissions
About this article
Cite this article
Ajmani, A., Shahnaz, T., Subbiah, S. et al. Hexavalent chromium adsorption on virgin, biochar, and chemically modified carbons prepared from Phanera vahlii fruit biomass: equilibrium, kinetics, and thermodynamics approach. Environ Sci Pollut Res 26, 32137–32150 (2019). https://doi.org/10.1007/s11356-019-06335-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06335-z